Science / Chemistry / Mathematics Texts

DOE Fundamentals Handbook - Material Science $13.95
The Mechanical Science handbook includes detailed explanations of operational terminology, as well as information on diesel engines, heat exchangers, pumps and valves. This book will provide YOU with a technical foundation essential for understanding the construction and operation of mechanical components such as Flywheels, Camshafts and Fuel Systems. The Mechanical Science handbook contains the information that YOU need about basic systems and equipment operations which are associated with various Hydrogen Science applications and experiments.

DOE FUNDAMENTALS HANDBOOK MATERIAL SCIENCE
VOLUMES 1-2
The Department of Energy (DOE) Fundamentals Handbooks consist of ten academic subjects, which include Mathematics; Classical Physics; Thermodynamics, Heat Transfer, and Fluid Flow; Instrumentation and Control; Electrical Science; Material Science; Mechanical Science; Chemistry; Engineering Symbology, Prints, and Drawings; and Nuclear Physics and Reactor Theory. The handbooks were first published as Reactor Operator Fundamentals Manuals in 1985 for use by DOE category A reactors. The subject areas, subject matter content, and level of detail of the Reactor Operator Fundamentals Manuals were determined from several sources and prepared by the DOE Training Coordination Program. Each handbook contains an abstract, a foreword, an overview, learning objectives, and text material, and is divided into modules.
The Material Science Handbook was originally developed to assist nuclear facility operating contractors in providing operators, maintenance personnel, and the technical staff with the necessary fundamentals training to ensure a basic understanding of the structure and properties of metals. The handbook includes information on the structure and properties of metals, stress mechanisms in metals, failure modes, and the characteristics of metals that are commonly used in DOE nuclear facilities. This information will provide personnel with a foundation for understanding the properties of facility materials and the way these properties can impose limitations on the operation of equipment and systems. The Material Science handbook presents enough information to provide the reader with a fundamental knowledge level sufficient to understand the advanced theoretical concepts presented in other subject areas, and to better understand basic system operation and equipment operations.
The Material Science handbook consists of five modules that are contained in two volumes. The following is a brief description of the information presented in each module of the handbook.
Volume 1 of 2
Module 1 - Structure of Metals
- Explanation- Explains the basic structure of metals and how those structures are effected by various processes. The module contains information on the various imperfections and defects that the metal may sustain and how they affect the metal.
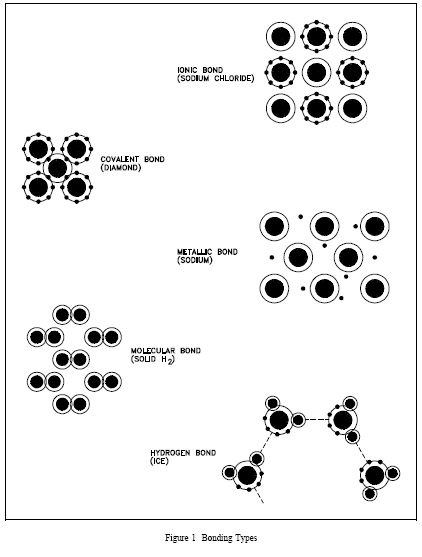
The types of bonds in a material are determined by the manner in which forces hold matter together. Figure 1 illustrates several types of bonds and their characteristics are listed below.
a. Ionic bond - In this type of bond, one or more electrons are wholly transferred from an atom of one element to the atom of the other, and the elements are held together by the force of attraction due to the opposite polarity of the charge.
c. Covalent bond - A bond formed by shared electrons. Electrons are shared when an atom needs electrons to complete its outer shell and can share those electrons with its neighbor. The electrons are then part of both atoms and both shells are filled.
d. Metallic bond - In this type of bond, the atoms do not share or exchange electrons to bond together. Instead, many electrons (roughly one for each atom) are more or less free to move throughout the metal, so that each electron can interact with many of the fixed atoms.
e. Molecular bond - When the electrons of neutral atoms spend more time in one region of their orbit, a temporary weak charge will exist. The molecule will weakly attract other molecules. This is sometimes called the van der Waals or molecular bonds.
f. Hydrogen bond - This bond is similar to the molecular bond and occurs due to the ease with which hydrogen atoms are willing to give up an electron to atoms of oxygen, fluorine, or nitrogen.
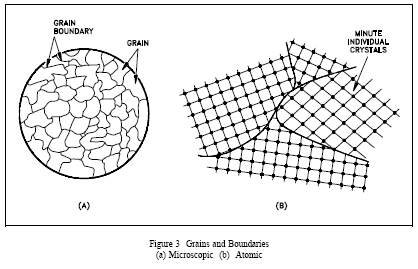
If you were to take a small section of a common metal and examine it under a microscope, you would see a structure similar to that shown in Figure 3(a). Each of the light areas is called a grain, or crystal, which is the region of space occupied by a continuous crystal lattice. The dark lines surrounding the grains are grain boundaries. The grain structure refers to the arrangement of the grains in a metal, with a grain having a particular crystal structure.
The grain boundary refers to the outside area of a grain that separates it from the other grains. The grain boundary is a region of misfit between the grains and is usually one to three atom diameters wide. The grain boundaries separate variously-oriented crystal regions (polycrystalline) in which the crystal structures are identical. Figure 3(b) represents four grains of different orientation and the grain boundaries that arise at the interfaces between the grains.
BONDING
Atomic Bonding
Order in Microstructures
Summary
COMMON LATTICE TYPES
Common Crystal Structures
Summary
GRAIN STRUCTURE AND BOUNDARY
Grain Structure and Boundary
Summary
POLYMORPHISM
Polymorphism Phases
Summary
ALLOYS
Alloys
Common Characteristics of Alloys
Type 304 Stainless Steel
Composition of Common Engineering Materials
Summary
IMPERFECTIONS IN METALS
Microscopic Imperfections
Macroscopic Defects
Summary
Module 2 - Properties of Metals
- Explanation- Contains information on the properties considered when selecting material for a nuclear facility. Each of the properties contains a discussion on how the property is effected and the metal's application.
STRESS
Definition of Stress
Types of Stress
Types of Applied Stress
Summary
STRAIN
Definition of Strain
Types of Strain
Deformation of Cubic Structures
Summary
YOUNG'S MODULUS
Hooke's Law
Young's Modulus (Elastic Modulus)
Summary
STRESS-STRAIN RELATIONSHIP
Elastic Moduli
Tensile (Load) Tests and Stress-Strain Curves
Summary
PHYSICAL PROPERTIES
Strength
Ultimate Tensile Strength
Yield Strength
Ductility
Malleability
Toughness
Hardness
How Alloys Affect Physical Properties
Summary
WORKING OF METALS
Heat Treatment
Cold and Hot Working
Summary
CORROSION
Corrosion
General Corrosion
Galvanic Corrosion
Localized Corrosion
Summary
HYDROGEN EMBRITTLEMENT
Concern
Sources of Hydrogen
Hydrogen Embrittlement of Stainless Steel
Hydrogen Embrittlement of Zirconium Alloys
Summary
APPENDIX A - TRITIUM/MATERIAL COMPATIBILITY
Concerns
Compatibility
Solubility in Metals
Permeability
Nonhydriding Metals
Hydriding Metals
Graphite
Glasses
Ceramics .. A-5Plastics, Elastomers, and Oils
Volume 2 of 2
Module 3 - Thermal Shock
-Explanation- Contains material relating to thermal stress and thermal shock effects on a system. Explains how thermal stress and shock combined with pressure can cause major damage to components.
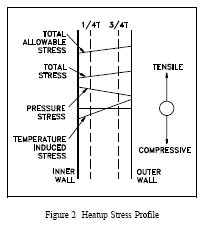
Stresses arising from coolant system pressure exerted against the inside vessel wall (where neutron fluence is greatest) are always tensile in nature. Stresses arising from temperature gradients across the vessel wall can either be tensile or compressive. The type of stress is a function of the wall thickness and reverses from heatup to cooldown. During system heatup, the vessel outer wall temperature lags the inner wall temperature. The stresses produced by this temperature gradient and by system pressure will produce the profile shown in Figure 2.
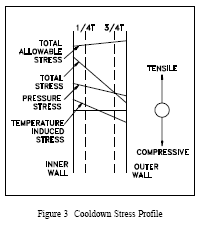
During system cooldown, the stress profile of Figure 3 is obtained. During cooldown, the outer wall lags the temperature drop of the inner wall and is at a higher temperature. It can be seen that during cooldown, the stresses at the 3/4 T location are tensile due to system pressure and compressive due to the temperature gradient. Thus during cooldown, the stresses at the 3/4 T location tend to cancel. At the 1/4 T location, however, the pressure and temperature stresses are both tensile and reinforce each other. Thus, the 1/4 T location is limiting during system cooldown.
THERMAL STRESS
Thermal Shock
Summary
PRESSURIZED THERMAL SHOCK
Definition
Evaluating Effects of PTS
Locations of Primary Concern
Summary
Module 4 - Brittle Fracture
-Explanation- Contains material on ductile and brittle fracture. These two fractures are the most common in nuclear facilities. Explains how ductile and brittle fracture are effected by the minimum pressurization and temperature curves. Explains the reason why heat-up and cool-down rate limits are used when heating up or cooling down the reactor system.
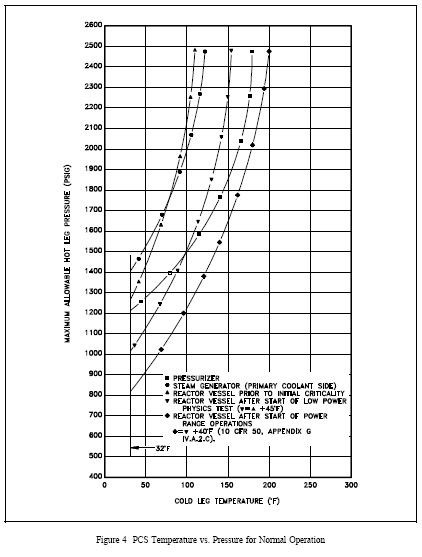
Minimum pressurization-temperature (MPT) curves specify the temperature and pressure limitations for reactor plant operation. They are based on reactor vessel and head stress limitations and the need to preclude reactor vessel and head brittle fracture. Figure 4 shows some pressure-temperature operating curves for a pressurized water reactor (PWR) Primary Coolant System (PCS).
Note that the safe operating region is to the right of the reactor vessel MPT curve. The reactor vessel MPT curve ensures adequate operating margin away from the crack arrest curve discussed above. The curves used by operations also incorporate instrument error to ensure adequate safety margin. Because of the embrittling effects of neutron irradiation, the MPT curve will shift to the right over core life to account for the increased brittleness or decreased ductility. Figure 4 also contains pressurizer and steam generator operating curves. Operating curves may also include surge line and primary coolant pump operating limitations. The MPT relief valve setting prevents exceeding the NDT limit for pressure when the PCS is cold and is set below the lowest limit of the reactor vessel MPT curve.
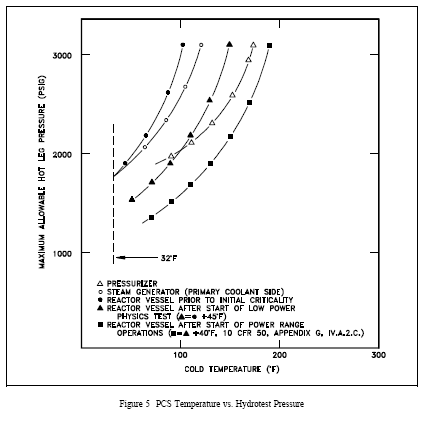
If the limit of the MPT curve is exceeded during critical operation, the usual action is to scram the reactor, cool down and depressurize the PCS, and conduct an engineering evaluation prior to further plant operation. During hydrostatic testing, minimum pressurization temperature precautions include making sure that desired hydrostatic pressure is consistent with plant temperatures so that excessive stress does not occur. Figure 5 shows MPT curves for hydrostatic testing of a PWR PCS. The safe operating region is to the right of the MPT curves. Other special hydrostatic limits may also apply during testing.
BRITTLE FRACTURE MECHANISM
Brittle Fracture Mechanism
Stress-Temperature Curves
Crack Initiation and Propagation
Fracture Toughness
Summary
MINIMUM PRESSURIZATION-TEMPERATURE CURVES
MPT Definition and Basis
Summary
HEATUP AND COOLDOWN RATE LIMITS
Basis
Exceeding Heatup and Cooldown Rates
Soak Times
Summary
Module 5 - Plant Materials
-Explanation- Contains information on the commonly used materials and the characteristics desired when selecting material for use.
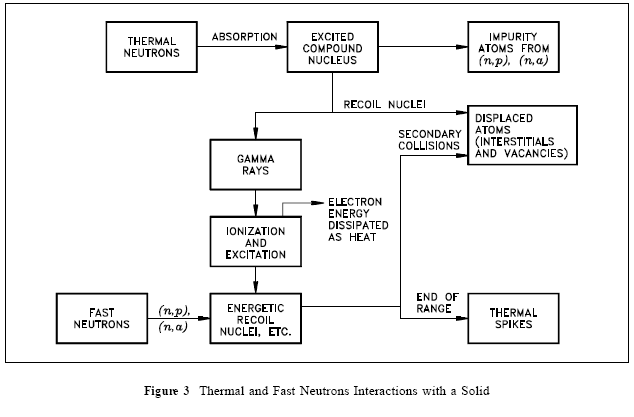
Atomic Displacements
If a target or struck nucleus gains about 25 eV of kinetic energy (25 eV to 30 eV for most metals) in a collision with a radiation particle (usually a fast neutron), the nucleus will be displaced from its equilibrium position in the crystal lattice, as shown in Figure 3.
PROPERTIES CONSIDERED WHEN SELECTING MATERIALS
Overview
Material Properties
Summary
FUEL MATERIALS
Overview of Material Types
Plutonium
Uranium
Thorium
Nuclear Fuel Selection
Summary
CLADDING AND REFLECTORS
Cladding
Reflector Materials
Summary
CONTROL MATERIALS
Overview of Poisons
Hafnium
Silver-Indium-Cadmium Alloys
Boron-Containing Materials
Summary
SHIELDING MATERIALS
Overview
Neutron Radiation
Gamma Radiation
Alpha and Beta Radiation
Summary
NUCLEAR REACTOR CORE PROBLEMS
Fuel Pellet-Cladding Interaction
Fuel Densification
Fuel Cladding Embrittlement
Effects on Fuel Due to Swelling and Core Burnup
Summary
PLANT MATERIAL PROBLEMS
Fatigue Failure
Work (Strain) Hardening
Creep
Summary
ATOMIC DISPLACEMENT DUE TO IRRADIATION
Overview
Atomic Displacements
Summary
THERMAL AND DISPLACEMENT SPIKES DUE TO IRRADIATION
Thermal Spikes
Displacement Spikes
Summary
EFFECT DUE TO NEUTRON CAPTURE
Effect Due to Neutron Capture
Physical Effects of Radiation
Summary
RADIATION EFFECTS IN ORGANIC COMPOUNDS
Radiation Effects
Summary
REACTOR USE OF ALUMINUM
Applications
Summary

DOE Fundamentals Handbook - Material Science $13.95
The Mechanical Science handbook includes detailed explanations of operational terminology, as well as information on diesel engines, heat exchangers, pumps and valves. This book will provide YOU with a technical foundation essential for understanding the construction and operation of mechanical components such as Flywheels, Camshafts and Fuel Systems. The Mechanical Science handbook contains the information that YOU need about basic systems and equipment operations which are associated with various Hydrogen Science applications and experiments.


