Chemistry

DOE Fundamentals Handbook - Chemistry $14.95
The Chemistry Handbook begins with a clear, concise introduction to the fundamental concepts of chemistry, including the atomic structure of matter. It goes on to discuss and explain the periodic table and the significance of the information in a periodic table, chemical bonding, the laws of chemistry and chemical equations. The handbook also includes information on the chemical interactions involved with corrosion processes, water chemistry control, including the principles of water treatment, the hazards of chemicals and gases and basic gaseous diffusion processes. This information will provide YOU with a foundation for understanding the chemical properties of materials and the way these properties can impose limitations or potentially cause disasters in planned experiments and serious projects. The information in the handbook is presented to provide a foundation for applying engineering concepts, including hydrogen energy applications. The Chemistry Handbook presents more than enough information to provide the reader with a fundamental knowledge level needed to understand more advanced theoretical concepts presented in many other scientific areas of study.

DOE FUNDAMENTALS HANDBOOK
CHEMISTRY
VOLUMES 1-2
The Department of Energy (DOE) Fundamentals Handbooks consist of ten academic subjects, which include Mathematics; Classical Physics; Thermodynamics, Heat Transfer, and Fluid Flow; Instrumentation and Control; Electrical Science; Material Science; Mechanical Science; Chemistry; Engineering Symbology, Prints, and Drawings; and Nuclear Physics and Reactor Theory. The handbooks were first published as Reactor Operator Fundamentals Manuals in 1985 for use by DOE category A reactors. The subject areas, subject matter content, and level of detail of the Reactor Operator Fundamentals Manuals were determined from several sources and prepared by the DOE Training Coordination Program. Each handbook contains an abstract, a foreword, an overview, learning objectives, and text material, and is divided into modules.
The Chemistry Handbook was originally developed to assist nuclear facility operating contractors in providing operators, maintenance personnel, and the technical staff with the necessary fundamentals training to ensure a basic understanding of chemistry. The handbook includes information on the atomic structure of matter; chemical bonding; chemical equations; chemical interactions involved with corrosion processes; water chemistry control, including the principles of water treatment; the hazards of chemicals and gases, and basic gaseous diffusion processes. This information will provide individuals with a foundation for understanding the chemical properties of materials and the way these properties can impose limitations on the operation of equipment and systems. The information in the handbook is presented to provide a foundation for applying engineering concepts. The Chemistry Handbook presents enough information to provide the reader with a fundamental knowledge level sufficient to understand the advanced theoretical concepts presented in other subject areas, and to better understand basic system and equipment operation.
The Chemistry handbook consists of five modules that are contained in two volumes. The following is a brief description of the information presented in each module of the handbook along with some selected figures included in the handbook.
Volume 1 of 2
Module 1 - Fundamentals of Chemistry
-Explanation- Introduces concepts on the atomic structure of matter. Discusses the periodic table and the significance of the information in a periodic table. Explains chemical bonding, the laws of chemistry, and chemical equations.
Appendix A - Basic Separation Theory
Introduces basic separation theory for the gaseous diffusion process. Discusses converter construction and basic operating principals.
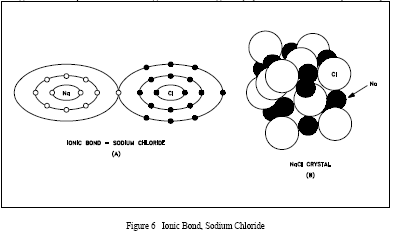
An ionic bond is formed when one or more electrons are wholly transferred from one element to another, and the elements are held together by the force of attraction due to the opposing charges. An example of ionic bonding is shown in Figure 6(A) for sodium chloride (table salt).
The sodium atom loses the one electron in its outer shell to the chlorine atom, which uses the electron to fill its outer shell. When this occurs, the sodium atom is left with a +1 charge and the chlorine atom a -1 charge. The ionic bond is formed as a result of the attraction of the two oppositely-charged particles. No single negatively-charged ion has a greater tendency to bond to a particular positively-charged ion than to any other ion.
CHARACTERISTICS OF ATOMS
Characteristics of Matter
The Atom Structure
Chemical Elements
Molecules
Avogadro’s Number
The Mole
Mole of Molecules
Summary
THE PERIODIC TABLE
Periodic Table
Classes of the Periodic Table
Group Characteristics
Atomic Structure of Electrons
Summary
CHEMICAL BONDING
Chemical Bonding
Ionic Bonds
Covalent Bonds
Metallic Bonds
Van der Waals Forces
Organic Chemistry
Alkanes
Alkenes
Alkynes
Aromatics
Alcohols
Aldehydes
Basic Chemical Laws
Forming Chemical Compounds
Combining Elements
Summary
CHEMICAL EQUATIONS
Le Chatelier’s Principle
Density
Molarity
Normality
Parts per Million
Chemical Equations
Balancing Chemical Equations
Summary
ACIDS, BASES, SALTS, AND pH
Acids
Bases
Salts
pH
pOH
Dissociation Constant
Summary
APPENDIX A BASIC SEPARATION THEORY
Introduction
Isotopic Separation
Separation Factor
Stage Separation
Barrier Measurements
Cascade Theory
Circuit Balance
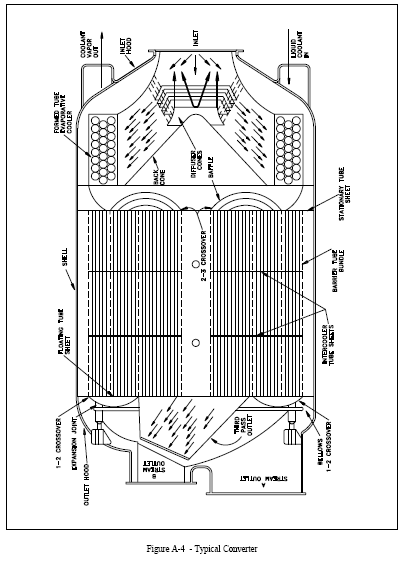
Converter Construction
Externally, the converter resembles a large cylindrical tank resting on its side (see Figure A-4). There are openings at each end for the necessary piping connections. The outlet end of the converter contains the "A" and "B" stream process gas outlets. The other end contains the mixed process gas inlet and the stage coolant inlet and outlet. The cylindrical tank is called the shell and is constructed of steel with welded joints. Its internal surface is nickel plated. There are three external reinforcing flanges around the outside of the shell. The many hundreds of barrier tubes, or tube bundles, contained in one converter are held in place by the spool, or spool piece. The spool consists of struts and a central tube, or core, which is perforated and allows part of the "A" stream leaving the barrier tubes to reach the converter outlet. On either end of the core are tube sheets into the holes of which the barrier tubes are sealed by rolling or swagging. Tube sheets are also mounted on the core between the end tube sheets to support the barrier tubes.
CONVERTERS
Converters
Converter Construction
The Gas Cooler
Barrier Tubing
Process Gas Flow
Diffusion
Module 2 - Corrosion
-Explanation- Supplies basic information on the chemical interaction taking place during the corrosion process between the environment and the corroding metal.
CORROSION THEORY
Corrosion
Electrochemical Cells
Oxidation-Reduction Reactions
Passivity and Polarization of Metal
Summary
GENERAL CORROSION
Conditions Contributing to General Corrosion
Corrosion of Iron
Factors Affecting General Corrosion Rate
Prevention Chemistry Control
Corrosion of Aluminum
Summary
CRUD AND GALVANIC CORROSION
Crud
Galvanic Corrosion
Prevention of Galvanic Corrosion
Summary
SPECIALIZED CORROSION
Pitting and Crevice Corrosion
Stress Corrosion Cracking
Summary
Volume 2 of 2
Module 3 - Reactor Water Chemistry
-Explanation- Describes the chemical measures taken to retard the corrosion often found in water systems. The consequences of radioactivity on facility cooling water systems are also addressed.
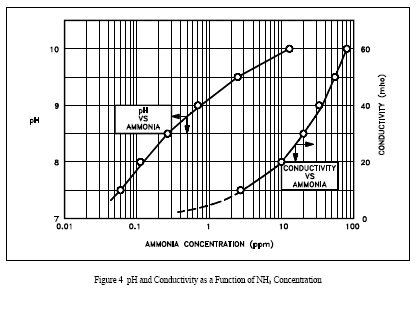
Conductivity
Conductivity of reactor facility water is measured to provide an indication of dissolved ionic substances in the coolant. Conductivity measurements provide quantitative rather than qualitative information because it is possible to determine the total conductivity of the ions present, but not the specific types of ions present. Because many ions such as iron (Fe+++), chromium (Cr+++), copper (Cu++) and aluminum (Al+++) are susceptible to forming oxides and plating out as scale on heat transfer surfaces, reactor coolant conductivity is normally controlled at a level as low as practicable and consistent with pH. By monitoring conductivity levels in the reactor facility systems, the operator is able to cross check the chemistry of these systems, thereby achieving a higher confidence level in the parameters measured. Regardless of the operating limits specified for a given reactor facility, operating relationships can be established between pH and conductivity levels of the coolant. Figure 4 shows a typical relationship of the pH and conductivity of a reactor coolant system using high pH, ammonium hydroxide chemistry control as a function of the ammonia (NH3) concentration.
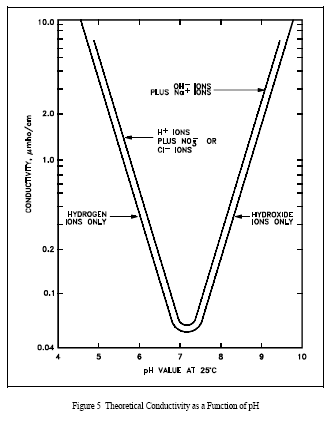
Figure 5 shows the theoretical relationship of pH versus conductivity in a solution containing pure water. A second curve is added to the graph that illustrates the relationship that exists when nitric acid (HNO3) is used as a pH control agent (such as may be utilized in facilities with aluminum components).
In both Figure 4 and Figure 5, a definite relationship exists between pH and conductivity, assuming no foreign ions are present. A similar graph could be constructed for those facilities using cation resins of a different base such as lithium or barium.
EFFECTS OF RADIATION ON WATER CHEMISTRY (SYNTHESIS)
Interaction of Radiation
Summary
CHEMISTRY PARAMETERS
Specific Parameters
pH
Dissolved Oxygen
Hydrogen
Total Gas
Conductivity
Chlorides
Fluorine
Radioactivity
Tritium
Abnormal Chemistry Conditions
Injection of Air
Fuel Element Failure
Resin Overheating
Summary
Module 4 - Principles of Water Treatment
-Explanation- Details the principles of ion exchange in the context of water purity. Discusses typical water treatment methods and the basis for these methods.
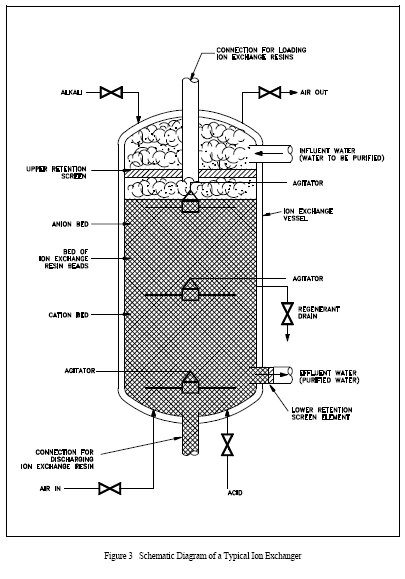
Because of the different densities of anion and cation resins, the flow of solution (impure water) is from top to bottom. If the flow were reversed, the lighter anion resin would gradually rise to the top by a process called classification, resulting in a layer of anion resin on top of the cation resin, as shown in Figure 3. In the example shown, the layering results from regeneration and/or backwash. In systems not using a backwash, the anion and cation resin beads are uniformly mixed. Many systems use a backwash procedure, if the resins are regenerated, to remove solids collected by filtration and to separate the resins for regeneration. They are remixed after regeneration.
For fixed amounts of anion and cation resins, the efficiency for removal of impurities is greater in a mixed-bed resin than a layered arrangement. The main reason is that for layered resins there may be large pH gradients within the column of resin. If, for example, the hydroxyl form resin is on top, as solution passes through it anionic impurities are removed and replaced by OR ions; thus, the pH increases. This increase in pH may decrease the efficiency in lower portions of the resin bed for removing impurities. It may also cause some impurities to precipitate because solubility changes with pH. The resin column will filter some undissolved material, but the efficiency for filtration is usually significantly less than that for removal by ion exchange. Thus, the overall efficiency is less than in a mixed-bed resin.
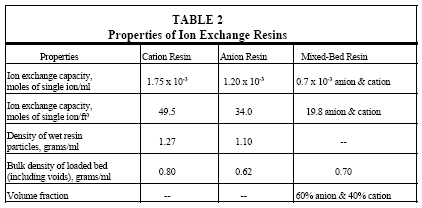
The capacity of ion exchange resins to remove impurity ions is given in Table 2 along with other information on resins. For instance, each cubic foot of a mixed-bed resin is capable of exchanging with 19.8 moles each of monovalent cations and anions. Mixed-bed resins are available commercially and in practical applications several cubic feet are used in a purification system.
PURPOSE OF WATER TREATMENT
Water Treatment
Summary
WATER TREATMENT PROCESSES
Principles of Ion Exchange
Specific Ion Exchanger Reactions
Summary
DISSOLVED GASES, SUSPENDED SOLIDS, AND pH CONTROL
Removal of Dissolved Gases
Removal of Suspended Solids
pH Control
Resin Bed Malfunctions
Summary
WATER PURITY
Water Purity
Summary
Module 5 - Hazards of Chemicals and Gases
-Explanation- Explains why certain chemicals are considered hazardous to facility personnel. Includes general safety rules on handling and storage.
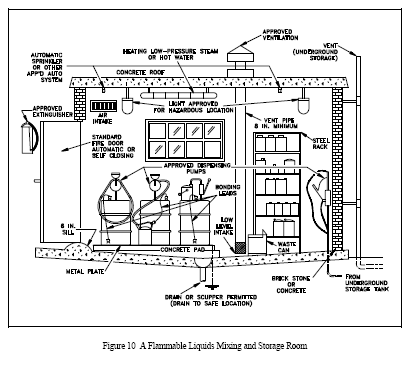
Losses by evaporation of liquid stored in safety cans at ordinary temperatures are negligible. Storage of flammable and combustible liquids in open containers should not be permitted. Approved containers for flammable liquids should be closed after each use and when empty. Warning labels should be removed from flammable liquid containers when empty (vapor free). Bulk Class I liquids should be stored in an underground (buried) tank or outside a building. No outlet from the tank should be inside a building unless it terminates in a special room, as illustrated in Figure 10.
CORROSIVES (ACIDS AND ALKALIES)
Acids
Alkalies
General Safety Precautions
Summary
TOXIC COMPOUND
Toxic Compounds
Summary
COMPRESSED GASES
Compressed Gases
Basic Safety Precautions Regarding Compressed Gases
Cryogenic Liquids
Treating Cold-Contact Burns
Specific Properties of Selected Industrial Gases
Hydrogen
Nitrogen
Oxygen
Sources of Ignition
Summary
FLAMMABLE AND COMBUSTIBLE LIQUIDS
Flammable and Combustible Liquids Definitions
Safety Precautions
Storage
Summary

DEPARTMENT OF ENERGY PRIMER ON
LEAD-ACID STORAGE BATTERIES
The Department of Energy Primer on Lead-Acid Storage Batteries was originally prepared as an information resource for personnel responsible for operation of the Department's nuclear facilities. This Primer contains fundamental information that will be helpful to most personnel involved in lead-acid battery applications. The reader is reminded to always follow the manufacturer's directions and to consult the manufacturer for help in battery sizing and selection. This Primer is provided as an information resource intended to supplement battery safety or hazardous material training.
The Primer on Lead-Acid Storage Batteries contains an introduction and sections on the following topics:
Lead-Acid Battery Types
Operation and Construction
Applications
Sizing and Selection
Maintenance
Storage, Transportation, and Disposal.
TABLE OF CONTENTS
INTRODUCTION
The purpose of this Primer is to provide operation and maintenance personnel with the information necessary to safely operate and maintain lead-acid storage battery systems. There are many hazards associated with lead-acid battery operation including acid burn, fire, explosion, and electrical shock. An understanding of the operating principles and safety precautions for storage batteries will help prevent personal injury and damage to facilities.
Upon completion of this Primer the reader should be able to do the following:
· Identify the differences between primary and secondary batteries.
· Identify the major types of lead-acid storage batteries.
· Define the following terms: cell, battery, electrolyte, separator, terminal, electrode, thermal runaway, gassing.
· Identify the active materials in the lead-acid cell.
· Describe the effects of temperature and discharge rate on battery capacity and life.
· Identify industry and government standards for maintenance, testing, replacement, sizing, and installation of lead-acid batteries.
· Identify the three most common applications of lead-acid batteries.
· Identify and describe four charging techniques.
· Identify safety precautions for operating and maintaining lead-acid batteries.
· Identify federal regulations governing lead-acid battery disposal.
· Identify the two basic types of "maintenance-free" batteries.
· Describe the effect that overcharging has on gassing and thermal runaway
DEFINITIONS
BATTERY COMPONENTS AND OPERATION
Cells vs. Batteries
Primary and Secondary Cells and Batteries
Battery Components
Cell and Battery Voltage
Capacity and Battery Ratings
Series and Parallel Connections
LEAD-ACID BATTERY TYPES
Flooded Lead-Acid Batteries
Sealed Lead-Acid Batteries
OPERATION AND CONSTRUCTION
Lead-Acid Battery Active Materials
Electrochemistry of the Lead-Acid Cell
Negative and Positive Plate Construction Methods
Antimony/Calcium/Selenium/Tin Alloying
Specific Gravity
Effects of Discharge Rate and Temperature on Capacity and Life
APPLICATIONS
Starting, Lighting, and Ignition
Industrial
Traction
Stationary
Portable
SIZING AND SELECTION
MAINTENANCE
General
Matching the Charger to Battery Requirements
Avoiding Over-discharge
Maintaining Electrolyte Levels
Cleaning
Avoiding High Temperatures
Supplying an Equalizing Charge
Safety Precautions
Testing
STORAGE, TRANSPORTATION, AND DISPOSAL
Storage
Transportation
Disposal
BIBLIOGRAPHY
CONCLUDING MATERIAL
LIST OF FIGURES
Figure 1. Major components of a cell
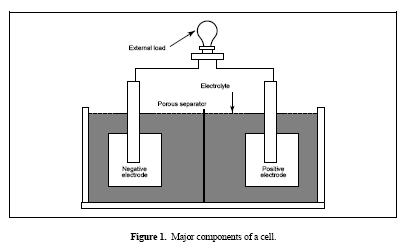
Figure 2. Cells connected in series
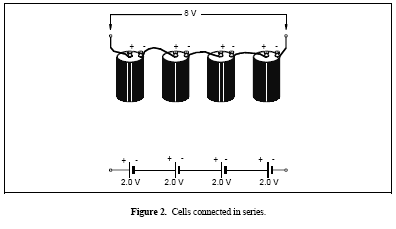
Cells and batteries may be connected in series, parallel, or combinations of both. Cells or batteries connected in series have the positive terminal of one cell or battery connected to the negative terminal of another cell or battery. This has the effect of increasing the overall voltage but the overall capacity remains the same. For example, the 12-V lead-acid automobile battery contains 6 cells connected in series with each cell having a potential difference of about 2 V. Another example of cells or batteries connected in series is shown in Figure 2.
Figure 3. Cells connected in parallel
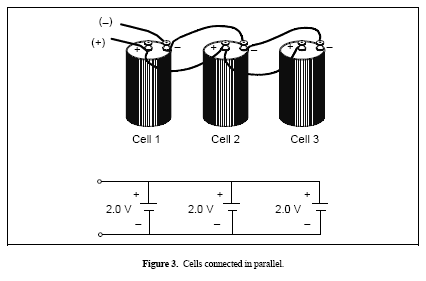
Cells or batteries connected in parallel have their like terminals connected together. The overall voltage remains the same but the capacity is increased. For example, if two 12-V automotive batteries were connected in parallel, the overall voltage for the batteries would still be 12 V. However, the connected batteries would have twice the capacity of a single 12-V battery. Another example of cells or batteries connected in parallel is shown in Figure 3.
Figure 4. Typical plant é plate
Figure 5. Typical construction of a pasted plate grid
Figure 6. Typical construction of a tubular plate
Figure 7. Self-discharge rates of three grid materials
Figure 8. Changes in voltage and specific gravity during charge and discharge
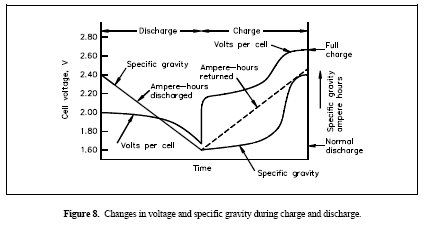
Specific Gravity
One of the key parameters of battery operation is the specific gravity of the electrolyte. Specific gravity is the ratio of the weight of a solution to the weight of an equal volume of water at a specified temperature. Specific gravity is used as an indicator of the state of charge of a cell or battery. However, specific gravity measurements cannot determine a battery's capacity. During discharge, the specific gravity decreases linearly with the ampere-hours discharged as indicated in Figure 8.
Figure 9. Typical effects of discharge rate on battery capacity
Figure 10. Typical effects of depth of discharge on traction battery life
Figure 11. Typical effects of operating temperatures on traction battery capacity
Figure 12. Cutaway view of a typical battery used for starting, lighting, and ignition (SLI)
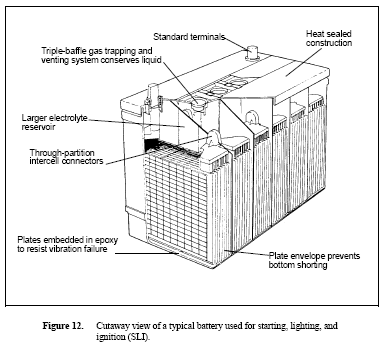
Starting, Lighting, and Ignition
SLI batteries are used by most people every day and are produced in greater numbers than any other type of lead-acid storage battery. These are used to start automobiles and most other kinds of internal combustion engines. They are not suitable for deep discharge applications, but excel for uses needing a high current for a brief time. They are usually charged in a "partial float" manner, meaning that the battery only receives a float charge while the vehicle is running. A cutaway view of a typical SLI battery is shown in Figure 12. SLI batteries are usually of the flat pasted plate design.
Figure 13. Typical stationary battery used for backup power
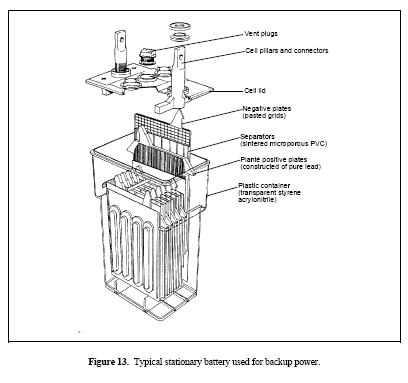
Stationary
Stationary batteries come in a wide variety of designs for different applications. They are used for applications where power is necessary only on a standby or emergency basis. Stationary batteries are infrequently discharged. Stationary batteries remain on a continuous float charge so that they can be used on demand. The largest types of stationary batteries are those used for electrical load leveling. Load-leveling batteries store electrical energy for times of peak power demand and are taken off-line during times of low power demand. Stationary batteries are also used for backup emergency power, telecommunications equipment, and uninterruptible power supplies. Stationary batteries are manufactured in a variety of plate designs. An example of a stationary battery used for backup power is shown in Figure 13.
Figure 14. Components of sealed lead-acid cell
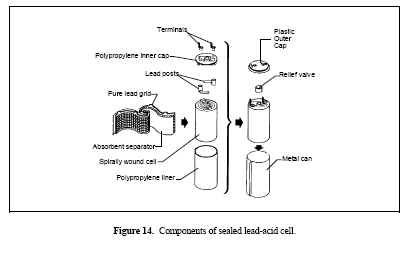
Portable
Portable lead-acid batteries are usually of the sealed type constructed similarly to that depicted in Figure 14. Their operation cannot usually be described as cyclic or float, but is somewhere in-between. Batteries in this category may be frequently deep cycled or remain unused for a relatively long time. Typical applications are portable tools, toys, lighting and emergency lighting, radio equipment, and alarm systems. Most portable batteries may be recharged to 80–90% of their original capacity in less than an hour using a constant-voltage charger.
Figure 15. Diagram of a duty cycle
Figure 16. Charge rate versus time for a typical constant-voltage charger

DOE Fundamentals Handbook - Chemistry $14.95
The Chemistry Handbook begins with a clear, concise introduction to the fundamental concepts of chemistry, including the atomic structure of matter. It goes on to discuss and explain the periodic table and the significance of the information in a periodic table, chemical bonding, the laws of chemistry and chemical equations. The handbook also includes information on the chemical interactions involved with corrosion processes, water chemistry control, including the principles of water treatment, the hazards of chemicals and gases and basic gaseous diffusion processes. This information will provide YOU with a foundation for understanding the chemical properties of materials and the way these properties can impose limitations or potentially cause disasters in planned experiments and serious projects. The information in the handbook is presented to provide a foundation for applying engineering concepts, including hydrogen energy applications. The Chemistry Handbook presents more than enough information to provide the reader with a fundamental knowledge level needed to understand more advanced theoretical concepts presented in many other scientific areas of study.
Includes DOE Fundamentals - Batteries For Free!


