Hydrogen Technology For Energy
 HYDROGEN TECHNOLOGY FOR ENERGY $29.95
HYDROGEN TECHNOLOGY FOR ENERGY $29.95
Hydrogen is attractive as a fuel of the future because it is abundant, relatively inexpensive and ecologically clean. Hydrogen gas can be burned in air, combining with oxygen to form water vapor, which eventually returns to the earth's water supply where it is again available as a source of fuel. Primary energy sources, e.g. nuclear or solar energy, can be utilized to produce hydrogen from water by electrolysis or thermochemical decomposition. The world's oceans, therefore, become vast reservoirs of a potential hydrogen source and the hydrogen itself becomes a means by which energy from the primary sources may be delivered to the consumer. However, the use of hydrogen as a universal fuel necessitates the development and implementation of efficient, standard methods for storing and handling it.
HYDROGEN TECHNOLOGY FOR ENERGY is primarily concerned with the technical aspects of storage and transmission systems for a proposed "hydrogen economy." Therefore, the various methods of producing hydrogen are not described in depth because they are important to the hydrogen economy only insofar as the hydrogen must be produced in large quantities at reasonable cost. The first chapter describes the hydrogen economy and suggests how it can be integrated into the energy system of the United States. The next three chapters are concerned with the technology of handling the various forms of hydrogen—gaseous, liquid and solid (in the form of metal hydrides). The final chapters describe some of the work which has been done or is underway in utilizing hydrogen as a fuel or energy storage system and delve into the safety, legal, political, socioeconomic and environmental implications of a hydrogen economy.
HYDROGEN TECHNOLOGY FOR ENERGY
Hydrogen is a most promising future medium for energy transmission and storage. It may be extracted from fossil fuels, from water or both simultaneously. While fossil fuel supplies must be regarded as a limited resource, water may be considered inexhaustible.
In addition to the raw material sources consumed in hydrogen production, energy sources must be used to extract hydrogen from fossil supplies or from water. This energy can be obtained directly from fossil fuels but primary energy sources including nuclear, solar, wind, fossil, geothermal, or ocean temperature gradients must be used to obtain hydrogen from water.
If hydrogen is to become a widely used energy carrier, it must be produced economically and efficiently on a large scale for a long time period. Therefore hydrogen production must be linked to those primary energy sources which are currently available, as well as to those which might be available in the future.
Hydrogen can be produced by current primary energy sources (for example, gas, oil, coal, fission) as well as by those energy sources which might be available in the future (i.e., fusion). Two important routes couple the several primary sources to hydrogen, thermochemical decomposition and electrolysis. Several primary sources could utilize either path (for example, nuclear or solar), while other sources only seem practical when coupled to one of these paths (for example, wind-electrolysis, geothermal-electrolysis).
An important characteristic of the future energy supply of the United States is that it will probably be based upon many primary sources. This trend is the result of the heavy demand on the limited domestic energy supplies. Economic and environmental constraints may cause energy to be extracted from many sources so that costs can be held down and the resulting pollutants (including reject heat) can be disposed of in many reservoirs. This book will guide you through Hydrogen Technology subject areas starting with the ways in which primary energy can be converted into hydrogen in ways that minimize economic and environmental costs.
INTRODUCTION
THE HYDROGEN ECONOMY
Primary Energy Sources
Solar Energy
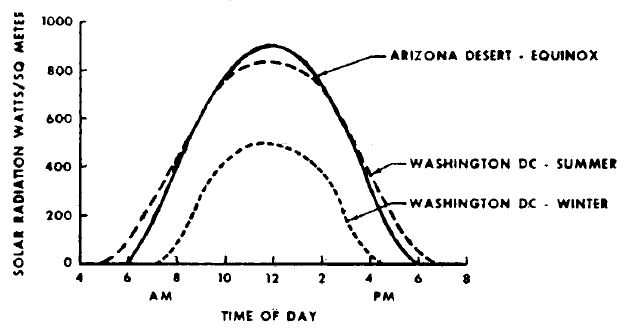
FIGURE 1.2: SOLAR ENERGY DENSITY AT TWO LOCATIONS
The amount of solar energy arriving at the outer edge of the earth's atmosphere is 2.0 calories per square centimeter minimum or 1,390 watts per square meter (1). Some of this is intercepted by the earth's atmosphere, and the amount of solar energy that finally impinges on the earth's surface depends upon the latitude, time of day, season of the year, and local weather conditions. These effects are illustrated in Figure 1.2. The top curve shows the solar radiation impinging on the desert floor near Yuma, Arizona, at the equinox (2). At noon, the energy flux is 920 w/m2, with the average being 700 w/m2 during the 12 hours of daylight. During the other 12 hours, the solar radiation is zero.
Some feeling for the effect of latitude and season can be obtained by considering conditions in Washington, D.C. On a clear summer day, the average intensity of the solar radiation striking the earth's surface during the 14 daylight hours is 478 w/m2 (3). During the winter, this value drops to an average of 248 w/m2 for 9.5 hours of daylight. The amount of energy impinging on one square meter in one day is the area under the curve in Figure 1.2. For the upper curve this quantity is 8.4 kwh, while the lower curve yields 2.36 kwh. The effects of geographic location and season are seen to be quite significant.
Wind Energy
Fossil Energy
Nuclear Energy
Miscellaneous
The Hydrogen Energy Conversion System
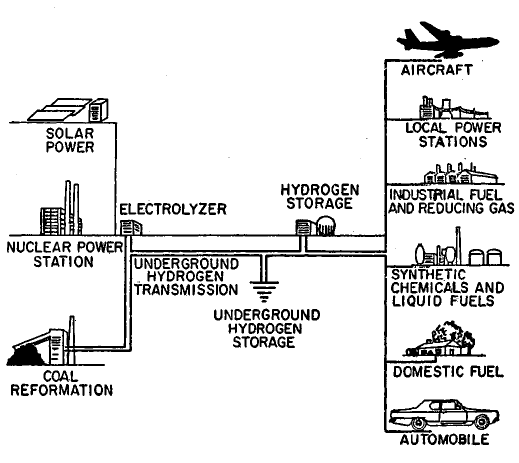
FIGURE 1.3: THE HYDROGEN ENERGY CONVERSION SYSTEM
If hydrogen were adopted as the main general-purpose synthetic fuel, the energy conversion system would probably have a configuration as illustrated in Figure 1.3 (29)(30). As the system grows, hydrogen would probably be produced by a variety of primary energy sources, initially by coal gasification and off-peak power from nuclear steam-electric generating plants, supplemented later by solar, geothermal, and fusion steam-electric generating plants. Perhaps in the more distant future, methods can be developed to produce hydrogen more directly from nuclear and solar heat by thermochemical reactions.
Hydrogen Production
Transportation and Storage of Hydrogen
Utilization of Hydrogen
Advantages and Disadvantages of Hydrogen as a Fuel
References
GASEOUS HYDROGEN
Comparison of Hydrogen Storage Methods
Existing Hydrogen Handling Systems
Economics of Transmission
A Hydrogen Pipeline System
Hydrogen Compatibility Problems
Types of Hydrogen Embrittlement
Embrittlement Mechanisms
Pressure Vessels and Pipelines for High Pressure Hydrogen
Aerojet Pressure Vessels
Los Alamos Hydrogen Pipeline
Fracture Mechanics Calculations
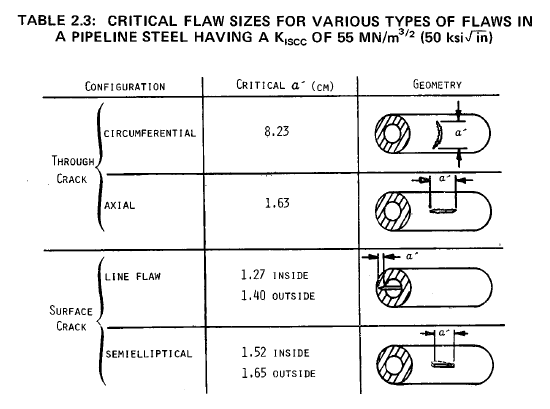
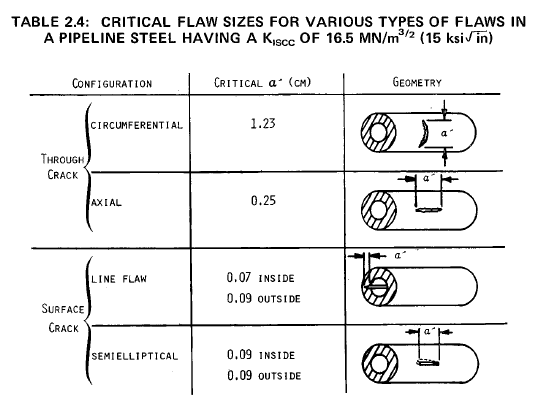
Compressor Requirements for Hydrogen Pipeline Transmission
Description of Existing Natural Gas Transmission System
Types of Ripeline Compressors
Reciprocating and Centrifugal Compressors
Regenerative Compressors
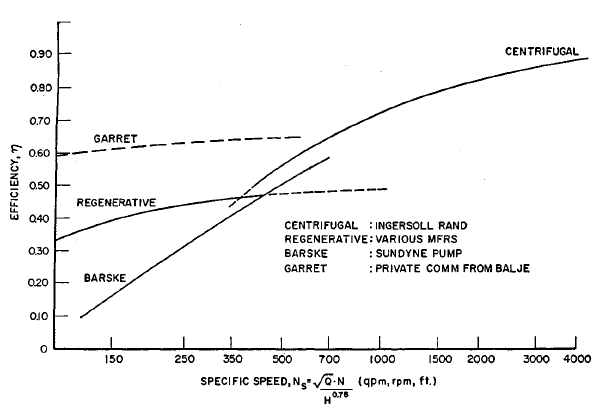
FIGURE 2.4: COMPARATIVE EFFICIENCY OF LOW SPECIFIC SPEED PUMPS
|
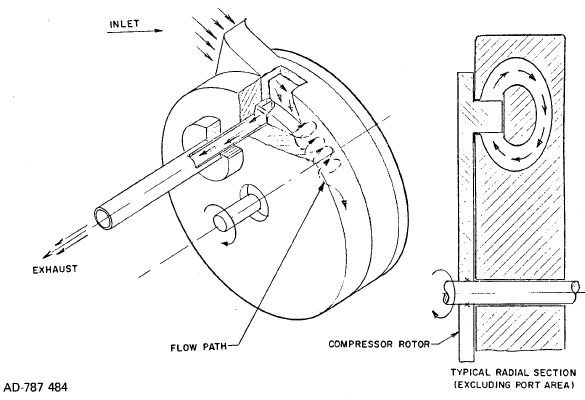
FIGURE 2.5: ADVANCE DESIGN REGENERATIVE TURBOMACHINE
|
| Developments carried out at Garret Company based on earlier work at Sunstrand Corporation carried out by Dubey under government sponsorship (31) have shown that it is possible to substantially improve the efficiency of regenerative turbomachinery by the improvement of the ordering of the flow pattern in the side channel, reduction in the extent of impeller blading and better aerodynamic design of the bladed elements. The improvement in efficiency obtainable is shown in Figure 2.4. Figure 2.5 (30)(31) shows a diagram of this improved type of regenerative turbomachine. Its now possible to contemplate the use of this design on hydrogen pipeline service, where high horsepower designs are needed. |
Cost of Compression Equipment for Hydrogen Storage
References
LIQUID HYDROGEN
Technology of Liquefaction and Storage
Liquefaction
Energy Requirements
Capital Costs
Liquefaction Costs
Recovery of Liquefaction Energy
Para-Hydrogen
Storage and Transfer
Representative Storage and Transfer Losses
Storage Dewars
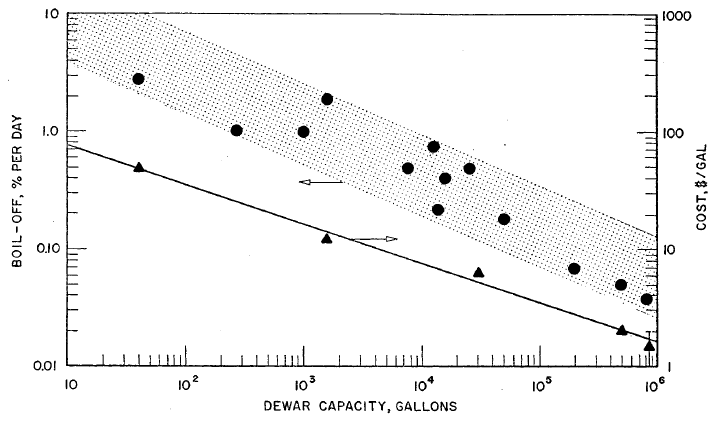
FIGURE 3.4: BOIL-OFF LOSSES AND CAPITAL COSTS FOR LIQUID HYDROGEN STORAGE DEWARS
Boil-off loss data and some cost data are plotted in Figure 3.4 versus tank capacity. These data indicate a rough trend for boil-off losses versus capacity; however, it is not surprising that the correlation is poor, since the vessel shape and type of insulation used varied widely. Detailed specifications for the various insulations used are not readily available so no attempt has been made to correlate subsets of the total body of data for specific types of insulation.
Transfer Systems
Storage and Transfer Problems
Safety
Fire and Explosion Hazards
Operation
Liquid Hydrogen Pumping
Pump Types
Technical Problems
The Role of Liquid Hydrogen in an Energy System
The Energy Pipe
Liquid Hydrogen-Cooled Electrical Transmission Cable
Superconducting Transmission Cable
Its superconducting transition temperature (Tc) is about 18K. Newer materials such as alloys of Nb, Al, and Ge, have ; values higher than 20K. To maintain superconducting properties in materials such as alloys of Nb, Al, and Ge,, coolants such as liquid helium (4K), slush hydrogen (14K), or a combination of the two must be used. There are economic advantages to using slush hydrogen, but the possible containment problem must again be examined.
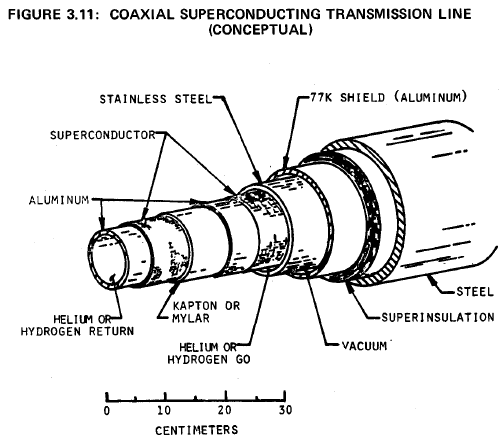
A drawing of one of several prototype designs is shown in Figure 3.11 (64). It is proposed that this design be used in a DC transmission line one kilometer long, with a power rating of five gigawatts. The superconducting material is plated onto the outer surfaces of two concentric aluminum tubes. Helium or slush hydrogen refrigerant will flow through the system at a pressure near one atmosphere. Surrounding the conductors and coolant are a stainless steel containment tube, a vacuum jacket, radiation shield, insulation and a structural steel case.
Peak Shaving Facility
Small Town Usage of Liquid Hydrogen
Comparison of Energy Transmission Costs for a Near-Urban Environment
References
SOLID HYDRIDES
Mechanism of Hydride Storage
Technical Problems of Hydride Storage
Heat Transfer
Deterioration
Safety
Steel Problems
Compressor
Metal Hydride Screening Experiments
Iron-Titanium-Manganese Alloy for Hydrogen Storage
Modelling Studies of Fixed-Bed Metal Hydride Storage Systems
Convection Bed Model
Conduction Bed Model
Results of Studies
High Efficiency Power Cycles Using Metal Hydride Compressors
Operation of an Iron-Titanium Bed as a Hydrogen Compressor
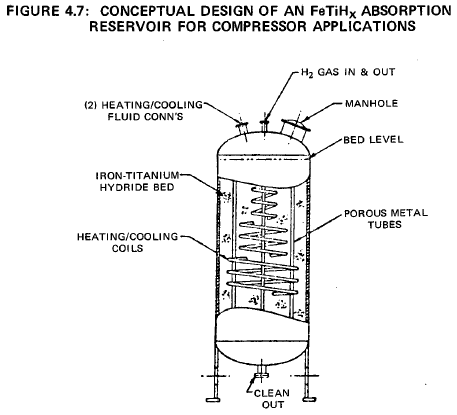
A simple design for a compressor would be a shell and tube heat exchanger, with the FeTiHx compound on the shell side and a coolant/heating bundle penetrating the granular bed. Hydrogen could be injected and removed from the bed through small diameter porous metal tubes dispersed in the bed and connected to a common header. Cooling would be by ambient temperature cooling water circulating through the tubes.
During H2 desorption hot water (or steam) from the low temperature heat source would circulate through the tubes. Figure 4.7 shows a conceptable design of an iron-titanium hydride compressor.
The pressure composition isotherms of the FeTiHx system generally exhibit some hysteresis. This results in some inefficiency in the cycle; the reaction is not completely reversible since the equilibrium charging pressure is slightly higher than the equilibrium discharge pressure. However, it is shown later that with available material this inefficiency is tolerable.
High and Low Temperature Heat Sources
Transmission of Hydrogen from Compressors to High Temperature Source
Implications of High Efficiency Power Cycle
Metal Hydrides for Ground Transport
References
USAGE OF HYDROGEN FOR FUEL AND ENERGY STORAGE
Hydrogen Utilization Devices
Water-Modified H2-02 (Aphodid) Burner
Hydrogen Fueled Internal Combustion Engine
Catalytic Burners
Flame Burners
Fuel Cells
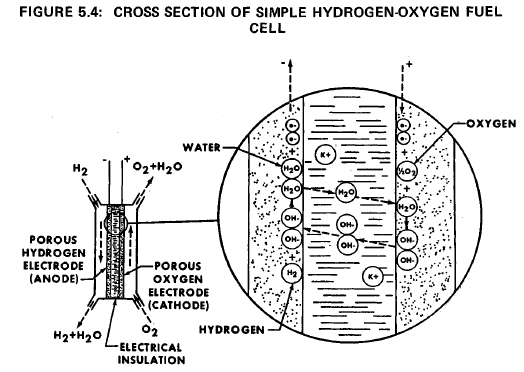
In principle, fuel cells are much like batteries; they are devices to transform chemical energy directly to electrical energy without using an engine as an intermediate step. Fuel cells differ from a battery in that the chemical energy input to the cell can be continuously replenished while a battery has a limited supply and once this supply is exhausted the battery is useless. The simplest fuel cell is a hydrogen-oxygen fuel cell as shown in Figure 5.4.
Other Devices
H2-02 Combustion Powered Steam-MHD Central Power Systems
Steam MHD System Performance
Capital and Operating Costs for Steam M HD Systems
Comparison of Power Systems
Advantages and Disadvantages
Operating Experiences with Hydrogen Fueled Reciprocating Engines
Hydrogen/Air Engines
Hydrogen/Oxygen Engines
Mixed-Fuel Hydrogen Engines
Energy Storage for Utilities-Brookhaven Optimization Model Studies
Elements and Cost of Hydrogen Storage Electric Peaking Plants
Cases Considered
Conclusions
Energy Storage for Utilities—PSE&G Maplewood Test System
Description of System
FIGURE 5.10: DIAGRAM SHOWING INTERNAL COMPONENTS OF RESERVOIR
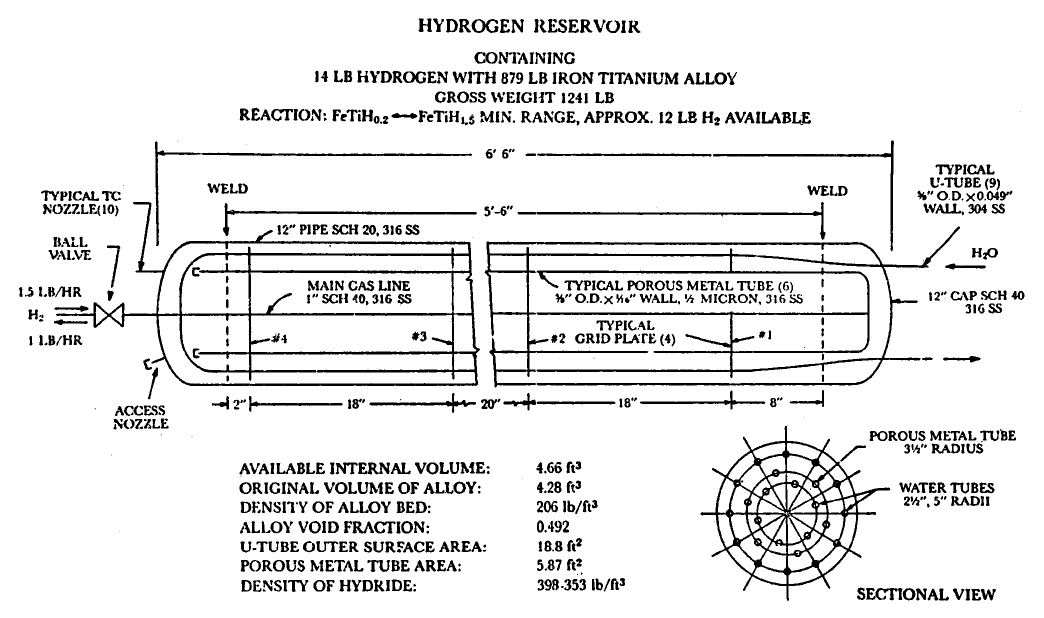
Conceptual Design of Metal Hydride Bed
Overall System Design Considerations
Economic Perspective
Fuel for Transportation Systems
Ground (Surface) Transportation
Water Transportation
Air (and Space) Transportation
Liquid Hydrogen System for Automotive Transportation
Vehicle Fuel Storage
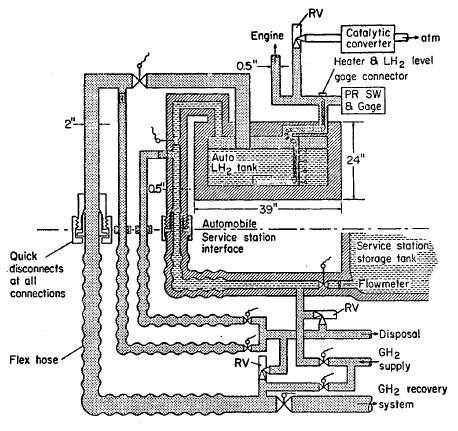
FIGURE 5.14: AUTOMOBILE DEWAR SYSTEM
A sketch of a car Dewar is given in Figure 5.14. The vehicle LH2 Dewar may be refilled or replaced at a service station whenever resupply is required. The number of connections for refilling the Dewar could be as many as five (electrical, LH2 supply, tank vent, vent line purge, and supply line purge) or as few as two (electrical and gas supply to engine) for replaceable Dewars. The number of connections should be minimized and reliable quick-disconnect type fittings should be developed to expedite refueling.
Hydrogen Production and Distribution System
Safety Considerations
Metal Hydride Storage System for Vehicles
Description of System
Safety Considerations
Weight, Economy and Range
Hydride-Dehydride Power System Useful for Refrigeration
References
NONTECHNICAL ASPECTS OF A HYDROGEN ECONOMY
Safety Implications
Legal Aspects
Political Aspects
Assessment of Safety Factors
Legal Implications
Energy Law
Regulatory Law
Environmental Law
International Law
Environmental Implications
Socioeconomic Implications
Social Costs and Values
Energy and the Social Future
Economic Costs
Hydrogen and Society
Political Implications
Politics and Energy
International Politics
Domestic Politics
References
HYDROGEN TECHNOLOGY EXPERTS
Hydrogen Properties
Heat Transfer
Hydrogen Production
Hydrogen Liquefaction Plants in the United States
Hydrogen Liquefaction and Purification
Ortho-Para Conversion
Slush Hydrogen
Gel Hydrogen
Metallic Hydrogen
Electrolysis Systems
Thermochemical Production of Hydrogen
Materials of Construction
Metals
Polymeric Composites
Insulations
Engineering Systems and Subsystems
Large Storage Tanks and Vessels (Field-Erected)
Vacuum-Jacketed Transfer Lines
Heat Exchangers
Liquid Hydrogen Pumps
Refrigerators
Compressors (Nonlubricated)
Hydrogen Disposal Systems
Transportation Systems for Hydrogen
Commercial Operations
NASA Operations
Regulatory Agencies
Advisory Agencies
Instrumentation for Hydrogen Systems
Temperature Measuring Instruments
Pressure Measuring Instruments
Flow Measuring Instruments
Liquid Level and Quantity Measuring Instruments
Combustible Gas Detectors
Hydrogen Applications
Rocket Propulsion (LH2-L02)
Hydrogen Fueled Aircraft
Hydrogen Superconducting Systems
Metal Hydrides
Hydrogen Fuel Cells
Internal Combustion Engines
Hydrogen Fuel, General
Safety and Hazards
Storage and Handling of Hydrogen
Electrical Codes and Equipment
Hydrogen Fires, Explosions and Spills
Hydrogen Safety and Accident Investigations
BIBLIOGRAPHY
HYDROGEN TECHNOLOGY FOR ENERGY $29.95
Hydrogen is attractive as a fuel of the future because it is abundant, relatively inexpensive and ecologically clean. Hydrogen gas can be burned in air, combining with oxygen to form water vapor, which eventually returns to the earth's water supply where it is again available as a source of fuel. Primary energy sources, e.g. nuclear or solar energy, can be utilized to produce hydrogen from water by electrolysis or thermochemical decomposition. The world's oceans, therefore, become vast reservoirs of a potential hydrogen source and the hydrogen itself becomes a means by which energy from the primary sources may be delivered to the consumer. However, the use of hydrogen as a universal fuel necessitates the development and implementation of efficient, standard methods for storing and handling it.
HYDROGEN TECHNOLOGY FOR ENERGY is primarily concerned with the technical aspects of storage and transmission systems for a proposed "hydrogen economy." Therefore, the various methods of producing hydrogen are not described in depth because they are important to the hydrogen economy only insofar as the hydrogen must be produced in large quantities at reasonable cost. The first chapter describes the hydrogen economy and suggests how it can be integrated into the energy system of the United States. The next three chapters are concerned with the technology of handling the various forms of hydrogen—gaseous, liquid and solid (in the form of metal hydrides). The final chapters describe some of the work which has been done or is underway in utilizing hydrogen as a fuel or energy storage system and delve into the safety, legal, political, socioeconomic and environmental implications of a hydrogen economy