Fuel Cell Technology

Fuel Cells For Public Utility And Industrial Power $29.95
FUEL CELLS FOR PUBLIC UTILITY AND INDUSTRIAL POWER contains a massive amount of practical, down-to-earth technical information relating to fuel cells for power plants. Fuel cell based power plants offer one of the most interesting possibilities for future power generation. The fuel cell is potentially more efficient than conventional plants and since the fuel reacts electrochemically rather than by combustion, there are far less air, thermal, and noise pollution issues. This book will inform you of the many advantages of Fuel Cells like the fact that they can be air-cooled and need not be adjacent to a body of water. It also points out important considerations for Fuel Cell public utility, such as the concept of modularity and efficiency considerations. With eight distinct sections ranging from “TYPES OF FUEL CELLS—THEIR OPERATION AND USE” and “ASSESSMENT OF FUELS FOR POWER GENERATION BY ELECTRIC UTILITY FUEL CELLS” to “FUEL CELLS FOR PUBLIC UTILITY APPLICATIONS—GENERAL” and “FUEL CELL POWER PLANT EVALUATION,” this book makes the advantages of small-scale fuel cell power units for smaller municipalities, large office complexes and shopping centers obvious.


Fuel Cells for Public Utility and Industrial Power
Although much of the interest in fuel cells is due to their efficient use of fuel, there are considerable pollution control advantages to be gained as well. Because the fuel reacts electrochemically rather than by burning in air, no nitrogen oxides are formed. For the same reason, emissions of unburned and partly burned gaseous and particulate products are essentially nil. The only moving parts in fuel batteries are fuel pumps and, perhaps, electrolyte pumps, so operation is inherently very quiet. There is relatively little thermal pollution because less energy is lost as heat.
The uses to which fuel cells may most profitably be applied are electric power generation and transportation. Most of the nonelectrical energy in the industrial sector, and nearly all in the commercial and residential sector is used for heating. Conversion of fuel to heat usually proceeds with high efficiency, so relatively little application of fuel cells in these sectors is seen. Because the fuel cells convert chemical energy directly to electrical energy, electrical power generation is probably their most natural application. While the output of each cell is low voltage dc power, cells may be connected in various series and parallel arrangements to give whatever voltage is desired, and large highly efficient inverters are available for conversion to ac.
A fuel cell system, unlike a heat engine, need not be big to be efficient. This characteristic, taken together with two others, low emissions and capability of operation on a variety of fuels, allows fuel cell systems to be operated almost anywhere. A small community power company can operate a power plant on the optimum fuel available locally with nearly the same efficiency achieved by a large central power station. A large metropolitan utility can disperse a number of generators throughout its area and match capacity to local demand, substantially reducing the expense and other problems associated with transmission and distribution of electricity.
In the transportation industry, the same virtues of efficiency and low pollution make the fuel cell attractive. Fuel cell systems of adequate performance to propel railroad trains, barges, and ships can probably be built with existing technology, at least, as far as cells themselves are concerned. All of the basic technology is available, the Fuel Cell power plant would be very smooth and quiet, virtually pollution-free, and could operate on conventional fuel –This book will tell you how!
INTRODUCTION
Advantages of Fuel Cells
Application of Fuel Cells
Choice of Fuels
Status of Fuel Cells
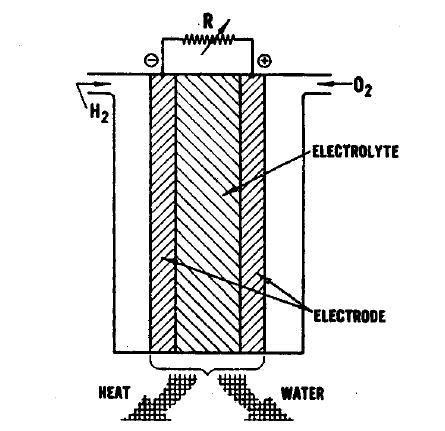
FIGURE 1.1: THE FUEL CELL
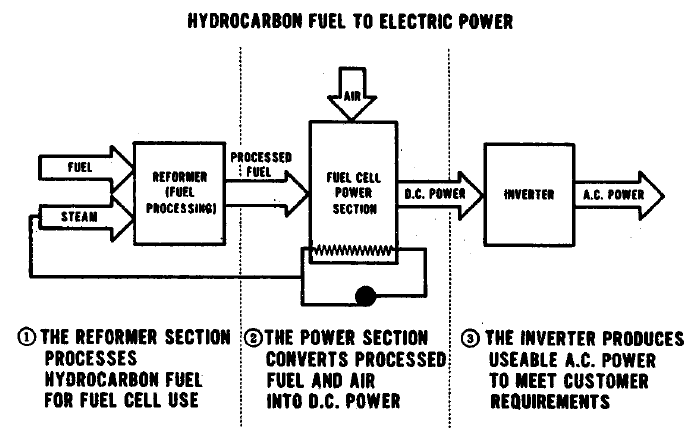
FIGURE 1.2: THE FUEL CELL POWER PLANT
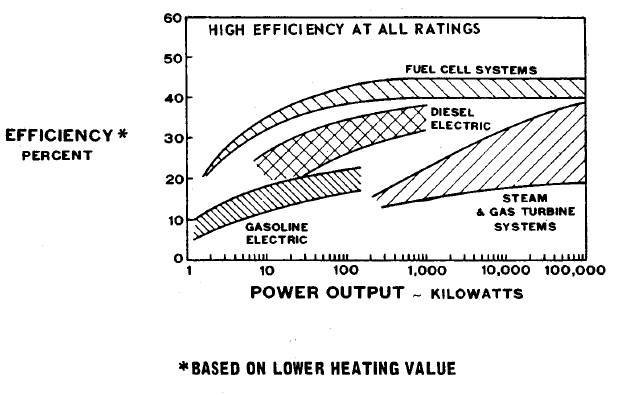
FIGURE 1.3: FUEL ECONOMY/EFFICIENCY FOR ALL SIZES
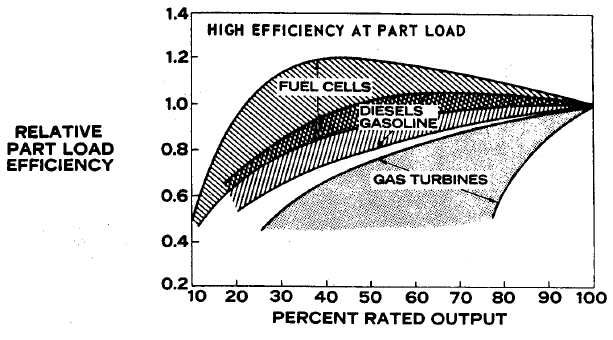
FIGURE 1.4: FUEL ECONOMY/EFFICENCY AT PART LOAD
TYPES OF FUEL ,CELLS—THEIR OPERATION AND USE
Introduction
Fuel Cell Operation
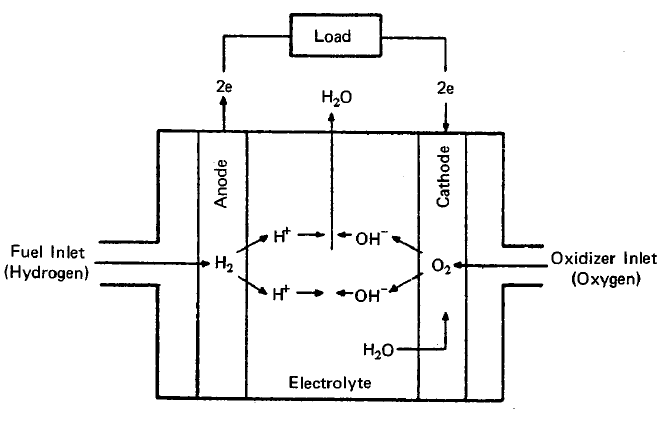 FUEL CELL OPERATION
FUEL CELL OPERATION
The essential features of a fuel cell (1)(2)(4)(5) are shown in Figure 2.1. The main components are a fuel electrode (anode), an oxidant or air electrode (cathode) and an electrolyte. In a typical application, the reactants are fed through the electrodes which are porous and are brought into contact with the electrolyte. Reactions take place which produce voltages at the electrodes. When an external load is connected, electrons are conducted through the load and perform useful work; whereas in the electrolyte, ions travel from one electrode to the other, completing the electrical circuit. Fuel cells continue to operate as long as the current flows through the load, as long as fuel and oxidant are supplied to the cell, and as long as structural and chemical integrity is maintained.
The principal reactions which occur in a hydrogen-oxygen fuel cell (2)(4) are shown in Figure 2.1. In this fuel cell hydrogen is fed through the anode where it reacts in the presence of a catalyst forming electrons and H+ ions, the latter passing into the electrolyte. At the other side of the fuel cell, the oxygen which is fed through the cathode acquires electrons and reacts with water from the electrolyte to form hydroxyl ions (OW). In this type of fuel cell, a solution of potassium hydroxide (KOH) is often used as the electrolyte. The Fl+ and the OH- ions are in equilibrium with water in the electrolyte. Electrons released at the hydrogen electrode pass through the external load circuit and are captured at the oxygen electrode. The electrical output from an individual cell is typically a direct current of 100 to 200 mA/cm2 at a voltage of about 1 volt (depending upon the reactants employed) (3)(11)(12). These cells can be connected in series and/or parallel to obtain a unit which will provide the appropriate voltage and current.
Fuel Cell Types
Direct Fuel Cells
Indirect Fuel Cells
Regenerative Fuel Cells
Fuels
Advantages
Efficiency
Pollution
Complexity
Scale
Special Applications
Space
Naval Propulsion
Electric Vehicles
Communication Systems
Commercial Systems
Other Applications
Fuel Cell Power Plants
System Characteristics
Applications
Future Systems
Economics
Major Commercial Research and Development Activities
P&WA/Gas Utilities
P&WA/Electric Utilities
Esso-Alsthom
Westinghouse
Status
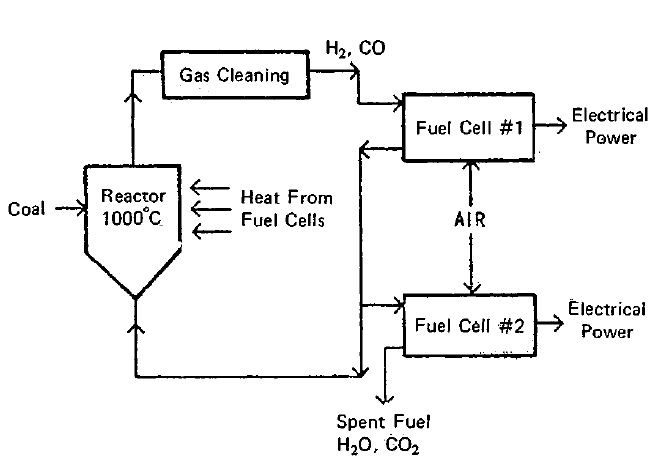
FIGURE 2.7: COAL-GASIFICATION FUEL CELL POWER PLANT
A schematic diagram of the Westinghouse coal-gasification fuel cell power system (8)(16) is given in Figure 2.7. This is a complete power system in that it includes a means for gasifying the coal as well as a means for producing electrical power. The heat released in the fuel cell is used to supply the heat required for the gasification process. For efficient heat transfer the fuel cells are immersed within the reactor. In operation, coal is fed to a fluidized reactor where it reacts with a partially oxidized fuel stream coming from one bank of fuel cells. The resulting fuel gas, after a cleaning process, is rich in H2 and CO. This fuel gas is fed to the fuel cells. The second bank of fuel cells is used to completely oxidize the fuel.
Near-Future Systems
Conclusions
References
ASSESSMENT OF FUELS FOR POWER GENERATION BY ELECTRIC UTILITY FUEL CELLS
Conversion Technology
Summary
Initial Process Screening
Later Process Screening
Secondary Fuels
Gasification Technology
Central Alternatives
On-Site Alternatives
Conceptual Fuel Supply Systems
Summary
Modular Approach
Basis for Evaluation
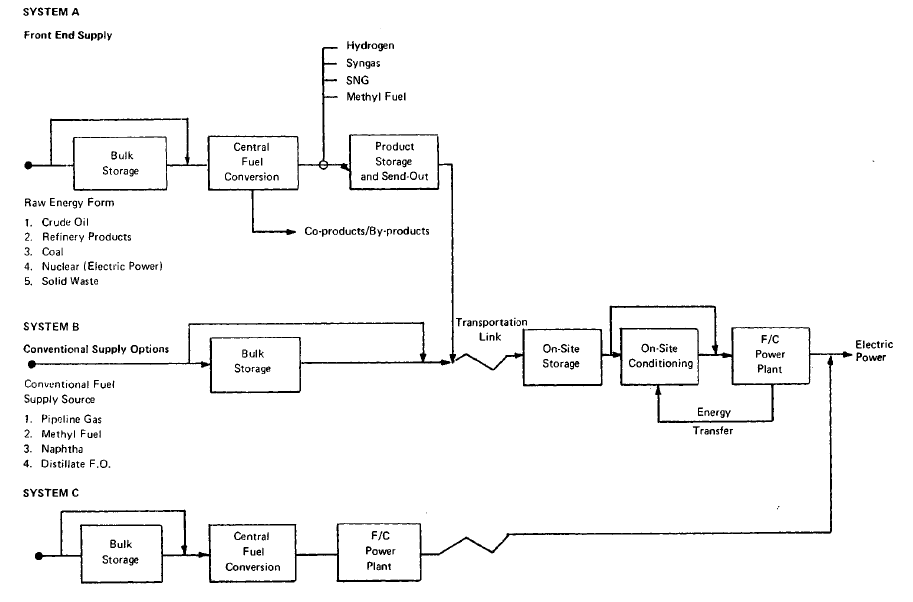
FIGURE 3.1: FUEL CELL POWER PLANT FUEL SUPPLY SYSTEMS
MODULAR APPROACH
In developing fuel cell fuel supply systems, three basic approaches were considered. The first, referred to as System A, consists of converting raw fuel at a central conversion facility and distributing a clean product fuel to dispersed fuel cell power plants.. ,,System B involves the delivery of purchased hydrocarbon feedstocks directly to the dispersed power plants where they are converted on-site to fuel cell .grade fuels. System C involves integrating a central station fuel cell with a central coal gasifier to eliminate fuel transportation costs, to fully integrate-all,necessary conversion in a single plant, and to take advantage of the economics'ofrscale of a large base load system. The general system schematic for these three options is shown in Figure 3.1.
Central Fuel Conversion for Dispersed Fuel Cells
Synthesis Gas Treatment and Conversion Systems
Coal Gasification Systems
Solid Waste Gasification
Heavy Oil Partial Oxidation
Central Fuel Conversion Economics for Dispersed Fuel Cells
On-Site Fuel Conversion for Dispersed Fuel Cells
Process Integration Opportunities (Central/On-Site)
Dispersed Fuel Cell/Processor Integration Analysis
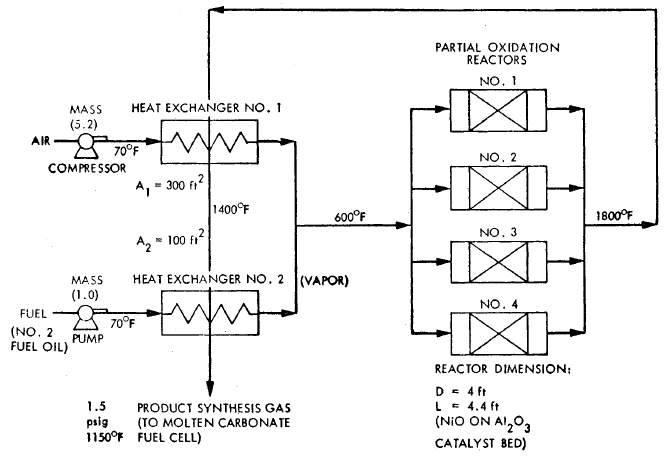
FIGURE 3.6: PROCESS FLOW DIAGRAM FOR A PARTIAL OXICATION PLANT - 26 MW
A process flow diagram for a catalytic partial oxidation fuel conditioner using distillate oil is shown in Figure 3.6 for synthesis gas production. No water is used in the process and carbon monoxide is not shift reacted to hydrogen, since the product gas is fed to a molten carbonate cell.
Capital Investment for On-Site Processors
Central Fuel Conversion for Central Fuel Cells
Process Integration Opportunities
Central Fuel Cell/Processor Integration Analysis and Economics
Recommendations for Future R&D Efforts
FUEL CELLS FOR PUBLIC UTILITY APPLICATIONS—WESTINGHOUSE STUDY
Summary
State of the Art
Aqueous Acid Fuel Cells
Alkaline Fuel Cells
Molten Carbonate Fuel Cells
Stabilized Zirconia Fuel Cells
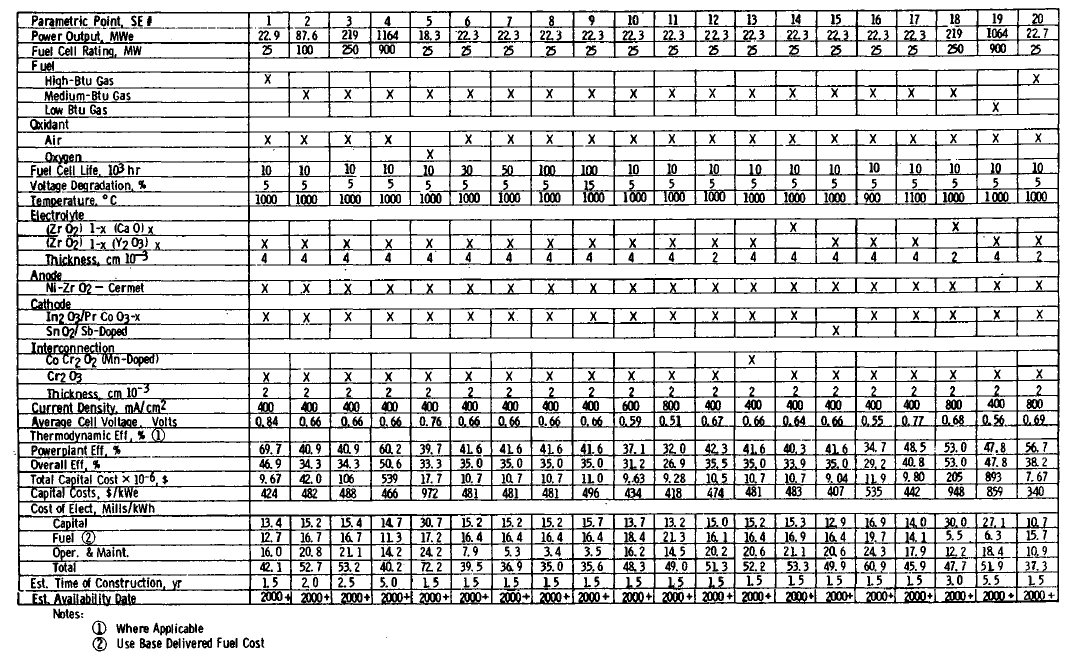
Description of Parametric Points
Phosphoric Acid Fuel Cell Power System
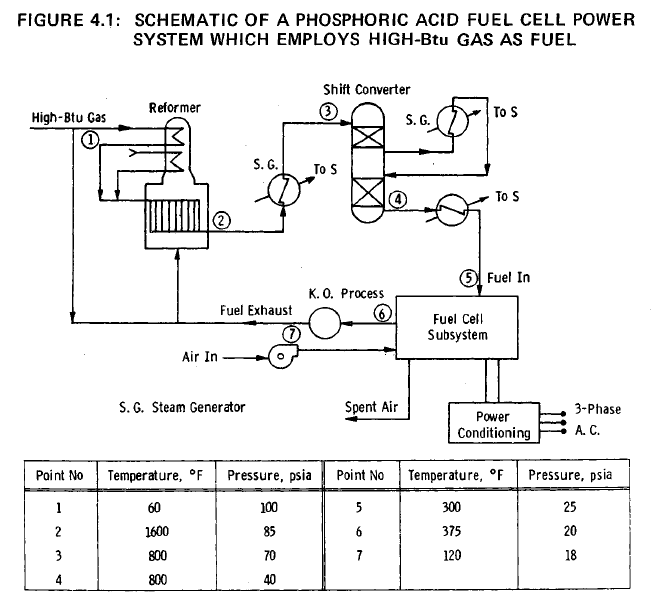
A schematic of the complete power system is shown in Figure 4.1. High-Btu gas from a 0.689 MPa (100 psi) abs line is assumed to be available, and is fed after preheating to a steam-methane reformer operating at a pressure of 0.689 MPa (100 psi) abs and a temperature of 1144°K (1600°F). The reformer effluent, consisting mainly of carbon monoxide, hydrogen, carbon dioxide and steam, is cooled and fed to a shift converter operating at 0.483 MPa (70 psi) abs and 700°K (800°F). The shift converter is operated at as low a temperature as possible in order to minimize the carbon monoxide concentration in the exit gas. The hydrogen-rich fuel gas is further cooled to approximately 422°K (375°F) and is fed to the ten fuel cell modules. Air is supplied to the modules by means of blowers, as shown in Figure 4.1.
Steam, required for the steam reformation of methane (the principal constituent of high-Btu gas) is raised in the cooling of the fuel gas between the reformer and the shift converter, in the shift converter, and between the shift converter and fuel cell subsystem. The water required for the steam generators is reclaimed from the fuel-gas exhaust by the knock-out process shown in Figure 4.1. The water-vapor depleted exhaust gases, containing approximately 10% of the hydrogen fed to the fuel cell modules, and the unused carbon monoxide, is mixed with a portion of the incoming high-Btu gas, and the mixture is burned to supply the heat required to cover the endothermic processes in the reformer. Further details of the fuel processing subsystem are provided in Appendix 1.
Alkaline Fuel Cell Power System
Molten Carbonate Fuel Cell Power System
Solid Electrolyte Fuel Cell Power System
Approach to. Efficiency Calculations
Phosphoric Acid Fuel Cell Power System
Alkaline Fuel Cell Power System
Molten Carbonate Fuel Cell Power System
High-Temperature Solid Electrolyte Fuel Cell
Capital, Site-Labor, and Operation and Maintenance Costs
Phosphoric Acid Fuel Cell Power System
Alkaline Fuel Cell Power System
Molten Carbonate Fuel Cell Power System
Solid Electrolyte Fuel Cell Power System
Results of Parametric Assessment
Phosphoric Acid Fuel Cell Power Systems
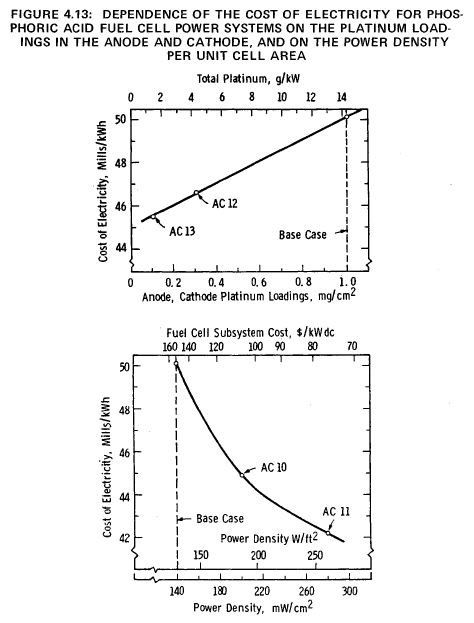
Figure 4.13 indicates that, provided a 0.56 mill/MJ (2 mills/kWh) penalty can be absorbed and an adequate supply of platinum is available, there is little point to efforts to reduce the electrode platinum loadings much below 0.4 mg/cm2 (8.2 x lb/ft2), corresponding to a platinum usage of approximately 6 g/kW (13.2 lb/MW). This conclusion is supported by Abens, Baker, DiPasquale and Michelko (29), who stated in a recent paper that "much obfuscation of cell costs has been caused by belaboring the advances made in catalyst cost reductions."
The power density, the product of the electrode current density and the cell voltage, has a marked effect on the COE, as shown by the data for AC10 and AC11 in Table 4.9. These results are also presented in Figure 4.13. A doubling of the base-case power density results in a 2.2 mills/MJ (7.9 mills/kWh) reduction in the COE. This graph may be used also to compute the effect of reductions in the selling price of the fuel cell subsystem because of the inverse relationship between the selling price and the power density.
Alkaline Fuel Cell Power System
Molten Carbonate Fuel Cell Power System
Solid Electrolyte Fuel Cell Power System
TABLE 4.12: VALUES OF ALL RELEVANT PARAMETERS FOR THE PARAMETRIC POINTS OF THE SOLID-ELECTROLYTE FUEL CELL POWER SYSTEM
|
Conclusions and Recommendations
Appendixes
Appendix 1—Fuel Processing for Low-Temperature Fuel Cell Power Plants
Appendix 2—Power-Conditioning Subsystem
Appendix 3—Oxygen Plants for Fuel Cell Power Systems
References
FUEL CELLS FOR PUBLIC UTILITY APPLICATIONS—GENERAL ELECTRIC STUDY
Fuel Cells—Low Temperature
Description of Cycle
Analytical Procedure and Assumptions
Design and Cost Basis
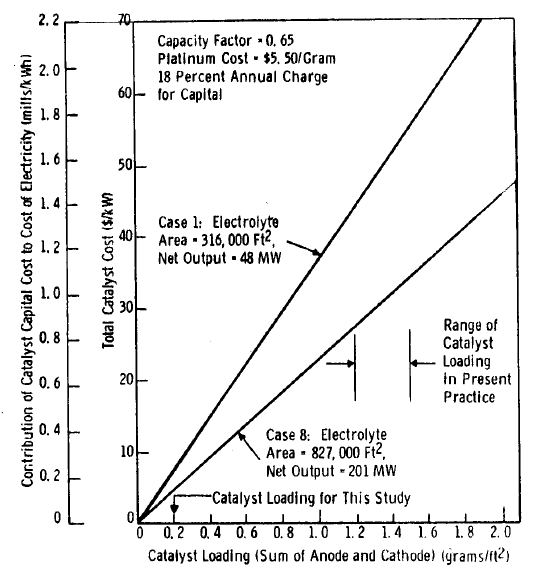
FIGURE 5.2: EFFECT OF CHANGE IN CATALYST LOADING
Inasmuch as the platinum loading of 0.2 g/ft2 has not been demonstrated experimentally but rather is a projection from present practice, it is useful to predict the effect of changes in catalyst loadings. Figure 5.2 shows the effect of loading on the catalyst capital cost and on cost of electricity. Two cases are shown. Case 1 is for a relatively low power density [net output: 152 W/ft2 (1640 W/m9] and Case 8 is for a high density [243 W/ft2 (2620 W/m2)]. From the figure it can be determined for Case 1 that an increase in loading from 0.2 to 1.2 g/ft2 will increase the cost of electricity by about 1.2 mills/kWh.
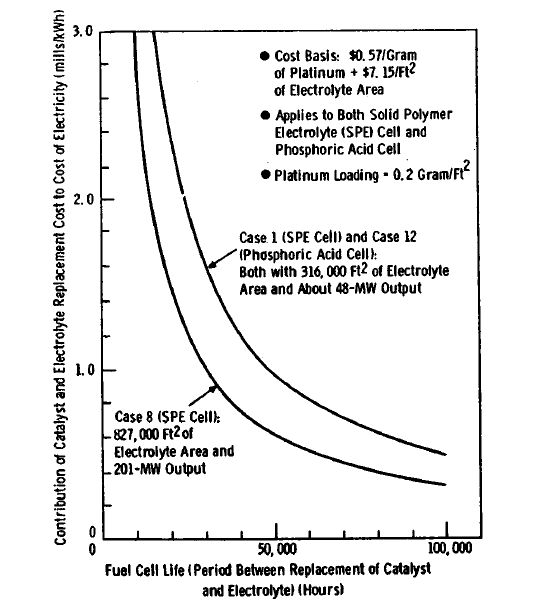
FIGURE 5.3: EFFECT OF FUEL CELL LIFE
In order to determine the effect of changes in replacement period, Figure 5.3 was prepared. This figure can be used to determine, for example, the cost effect of reducing the 100,000 hour period for Case 8 to some lower number, say to 30,000 hours. At a 30,000-hour replacement period, the contribution of catalyst and electrolyte replacement to the cost of electricity will rise to 1.0 mils/kWh, compared with 0.3 mils/kWh at the assumed period of 100,000 hours for Case 8.
Results
Fuel Cells—High Temperature
Description of Cycle
Analytical Procedure and Assumptions
Design and Cost Basis
Results
References
FUEL CELL POWER PLANT EVALUATION
Introduction
Low Temperature Fuel Cells
General Electric Treatment
Westinghouse Treatment
High Temperature Fuel Cells
General Electric Treatment
Westinghouse Treatment
Overall Comparison

FIGURE 6.5: ENERGY EFFICIENCY - COST OF ELECTRICITY MAP FOR VARIOUS TYPES OF FUEL CELL POWER PLANTS
OVERALL COMPARISON
With the aid of Figure 6.5 an overall comparison of the results of the fuel cell portion of the parametric study can be made. The G.E. low temperature fuel cell system points are divided into two groups to indicate the significant effect of hydrogen fuel. Likewise, the Westinghouse high temperature points are
divided into two groups to indicate the significant effect of using a steam bottoming cycle and/or integration with the gasifier. The general conclusions that can be drawn from inspection of Figure 6.5 are as follows:
(1) With low temperature fuel cell power systems the use of hydrogen fuel in place of H Btu gas improves efficiency and lowers the cost of electricity (COE).
(2) The Westinghouse estimates of low temperature fuel cell efficiencies (with no hydrogen fuel cases) were all higher than the G.E. overall efficiency estimates for H Btu/air. The Westinghouse estimates of COE for these same cases were either higher or lower than those of G.E.
(3) The results indicate that, in general, high temperature fuel cell systems are more efficient than low temperature fuel cell systems.
(4) Using a steam bottoming cycle and/or integration with the gasifier results in the highest efficiencies obtained with a high temperature fuel cell.
(5) The estimates by Westinghouse of the high temperature fuel cell efficiencies for systems with a steam bottoming cycle are significantly higher than a E.'s estimates.
Additional conclusions based on a closer examination of the results are discussed in the following section. In addition, the present general conclusions are more precisely stated and discussed. Finally, it is important to state that optimization was not a goal of Phase 1 of ECAS. Optimization could result in points with reduced COE
Assessment of Low Temperature Fuel Cells
General Electric Solid Polymer Electrolyte Systems
General Electric Phosphoric Acid Systems
Westinghouse Phosphoric Acid Systems
Westinghouse Alkaline (KOH) Systems
Assessment of High Temperature Fuel Cells
General Electric Zirconia Solid Electrolyte System
Westinghouse Zirconia Solid Electrolyte System
Westinghouse Molten Carbonate Systems
Significant Trends
Detailed Evaluation
Solid Polymer Electrolyte
Phosphoric Acid Electrolyte
Molten Carbonate Electrolyte
Zirconia Solid Electrolyte
Commercial Availability
Special Features of Fuel Cell Power Plants
Conclusions
Low Temperature Fuel Cell Systems
High Temperature Fuel Cell Systems
MARKETING CONSIDERATIONS
Summary
Historical Overview of Fuel Cells and Program
Fuel Cell Characteristics
Operating Characteristics
Environmental and Siting Characteristics
Response to Varying Load
Siting and Installation
Operation and Maintenance
Waste Heat
Areas of Application
Electric Utility Uses
Heat Recovery and Integrated Energy Systems Applications
Assumptions
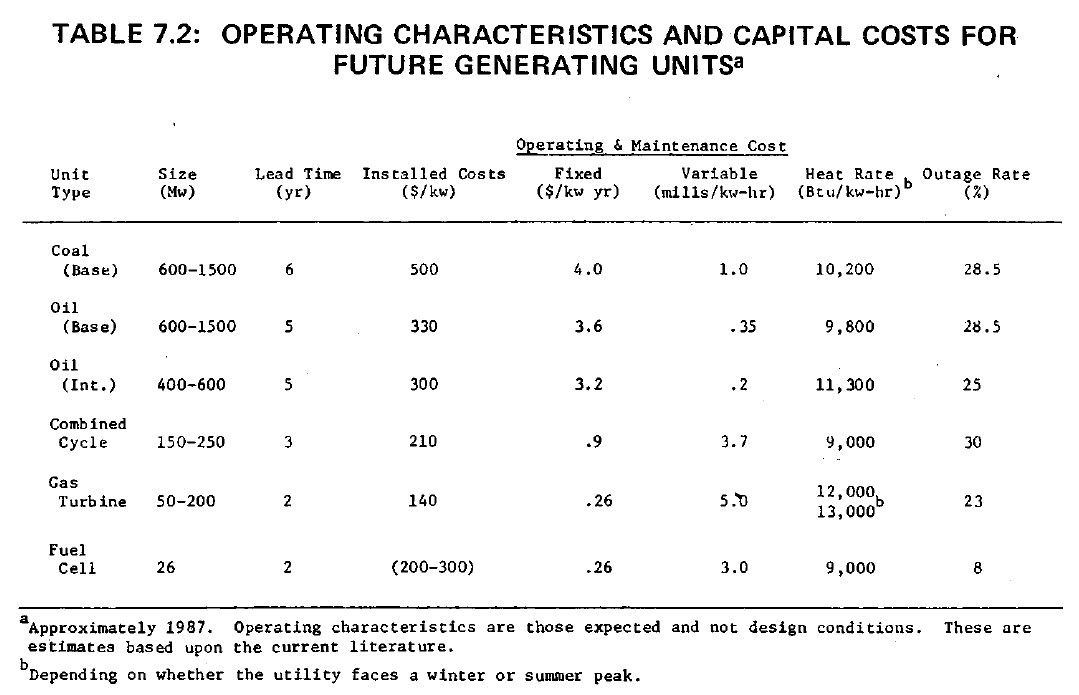
AREAS OF APPLICATION Electric Utility Uses
Fuel cells are clearly very well suited to peaking and intermediate uses in electric utilities, particularly where high efficiency and freedom from adverse environmental effects are desirable. Table 7.2 summarizes costs and efficiencies projected for peaking turbines, coal, and oil-fired intermediate generators, combined cycles, and fuel cells.
Benefit Analysis
Utility Applications
Integrated Energy Systems
Process Steam
Export Market
Conclusions
Appendixes
Appendix 1—Characteristics of Advanced Fuel Cell Types
Appendix 2—Effect of Electric Capacity on Fuel Cell Deployment
Appendix 3—Regional Fuel Prices 1985
Appendix 4—Regional Capacity Shares
Appendix 5—Annual Generating Cost per kW Based on 1987 Installation
References
PROPRIETARY PROCESSES

Fuel Cells For Public Utility And Industrial Power $29.95
FUEL CELLS FOR PUBLIC UTILITY AND INDUSTRIAL POWER contains a massive amount of practical, down-to-earth technical information relating to fuel cells for power plants. Fuel cell based power plants offer one of the most interesting possibilities for future power generation. The fuel cell is potentially more efficient than conventional plants and since the fuel reacts electrochemically rather than by combustion, there are far less air, thermal, and noise pollution issues. This book will inform you of the many advantages of Fuel Cells like the fact that they can be air-cooled and need not be adjacent to a body of water. It also points out important considerations for Fuel Cell public utility, such as the concept of modularity and efficiency considerations. With eight distinct sections ranging from “TYPES OF FUEL CELLS—THEIR OPERATION AND USE” and “ASSESSMENT OF FUELS FOR POWER GENERATION BY ELECTRIC UTILITY FUEL CELLS” to “FUEL CELLS FOR PUBLIC UTILITY APPLICATIONS—GENERAL” and “FUEL CELL POWER PLANT EVALUATION,” this book makes the advantages of small-scale fuel cell power units for smaller municipalities, large office complexes and shopping centers obvious.