|
|
Published by
www.KnowledgePublications.com
|
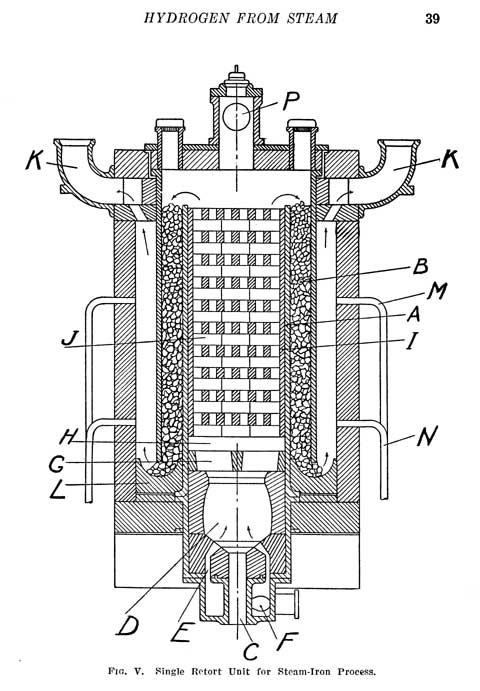
Original CONTENTS OF THE BOOK in BLUE with EXPLANATIONS and PHOTOS Inserted. CHAPTER I. INTRODUCTION . 15 Growth of the Industry. Uses of Hydrogen. Sources of Hydrogen Supply. Classification of Systems of Production. Choice of Process. Safety Precautions. - Explanation from Steve. Chapter one covers the history and use of hydrogen. Hydrogen has been used for several centuries on a regular basis. In the beginning it was used for balloons and other lighter than aircraft. Hydrogen has also EXTENSIVELY used in the metals field. For the reduction of a metal oxide to a metal. A good source of hydrogen makes it easy to make iron, zinc, aluminum and a whole variety of other metals. It is also used to increase the heat transfer inside a furnaces and as an isolation atmosphere. We have been able to produce hydrogen on large scales since the 1800's and even more so since the early 1900's. Only today in the 21st century is the general public so blind to the fundamentals of chemistry and the massive uses of hydrogen and views it with such tunnel vision. Hydrogen *IS* extensively made and used by all petroleum refineries. Some ways crude, others simple, some small and most are large. Chapter 2 below covers one of the ways that it is made in towers 100 feet tall. The same method ALSO works on a 4 foot tower which we will be demonstrating SOON on video snippets to you. This book *IS* very applicable to the experimenter, the hydrogen enthusiast and the student or researcher. CHAPTER II. HYDROGEN FROM STEAM AND IRON . . 25 Reactions of the Process. Historical. The Contact Mass. Typical Generator Units. Multi-retort Type. Single-unit Type. Operational Procedure. The Reduction Phase. The Steaming Period. Aeration. Thermal Balance of Process.
CHAPTER III. HYDROGEN FROM WATER-GAS AND
STEAM . 60 CHAPTER VII. HYDROGEN FROM AQUEOUS ALKALIS
. . 131 Covers the generation of hydrogen from
mostly organic sources. Including the dehydrogenation of
alcohol. Using " booze" as a source of Hydrogen by decomposing it
with heat and copper. Chapter also covers making
hydrogen by FERMENTATION. Yes! a special yeast that you can
still purchase that will decompose cellulose or corn or any of the
sugars. It produces flammable liquids that CAN be distilled
off to run your car with hydrogen boosting AND it also makes H2 and
CO2 in abundance. So much abundance that a company took these '
undesirable byproducts' and converted it under pressure into
methanol and sold the methanol. This makes millions of times more
hydrogen than 'blue green algae' Entire industries,
especially during WWII, were entirely dependent on this
method. This CAN be done in a 5 gallon container OR it can be
done in tanks that take up hundreds of thousands of gallons. How to purify hydrogen, clean it up, remove
sulfurs, how to test it etc.... great chapter. |
|
BUY NOW
$34.95 or less! |
To Purchase Industrial Hydrogen and/or the Hydrogen |
SUBJECT INDEX